![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is Science? |
A highly logical, systematic, and organized approach to problem solving. |
|
|
|
List the steps in the scientific method |
1) IDENTIFY the problem(purpose) 2) Propose a SOLUTION to the problem (hypothesis) 3) TEST the proposed solution through EXPERIMENTATION 4) Gather data using senses (observations) 5)ANALYZE the data using the brain 6)Formulate CONCLUSION (discussion) |
IPSTEGAF PHMO D |
|
|
What is Chemistry? |
A science which studies substances, their properties, structure, composition and transformations. |
PCST |
|
|
What are protons? |
smaller than atom, positive charge |
|
|
|
What are electrons? |
smaller than atom, Negative Charge |
|
|
|
What are neutrons? |
smaller than atom, neutral, no charge |
|
|
|
What are atoms? |
Neutral particles that consist of different proton, electron, and neutron combinations. |
|
|
|
What are cations? |
Positively Charged particles that are created when atoms lose electrons |
|
|
|
What are anions? |
Negatively charged particles that are created when atoms gain electrons. |
|
|
|
What are molecules? |
Larger particles that are created when atoms join together |
|
|
|
What is matter? |
anything that has mass and takes up space |
|
|
|
Pure substance |
Form of matter which is made of only ONE kind of particle or molecule |
|
|
|
The particle theory of Matter |
1) all matter is made of tiny particles 2) All particles of a pure substance are identical 3) the space between particles is large compared to the sizes of the particles themselves 4)Particles of matter are always moving 4) there are forces of attraction that exist between the particles of matter |
TILMA |
|
|
What is an element? |
An element is a pure substance which cannot be further broken down by ordinary chemical means. Since elements are pure substances they must be made up of only 1 kind of particle. The smallest particle of an element is called an atom. |
|
|
|
What is a compound? |
A compound is a pure substance which is made of 2 or more different types of elements. Since compounds are pure, they are also made of only 1 kind of particle. The smallest particle of a compound is a molecule. |
|
|
|
What is a compound? |
A compound is a pure substance which is made of 2 or more different types of elements. Since compounds are pure, they are also made of only 1 kind of particle. The smallest particle of a compound is a molecule. |
|
|
|
What is the composition of the following chemical formula? NaCl |
1 Na (sodium) atom joined (bonded) to 1 chlorine atom |
|
|
|
What is the composition of the following chemical formula? KMnO4 |
1 potassium atom joined to 1 Magnese atom and 4 oxygen atoms |
|
|
|
What is the composition of the following chemical formula? NH4OH |
1 nitrogen to 5 hydrogen to 1 oxygen |
|
|
|
What is the composition of the following chemical formula? Ca(OH)2 |
1 calcium to 2 hydrogen and 2 oxygen |
|
|
|
Bohr Rutherford Atomic Model |
1) the atom consists of mostly empty space 2) most of the mass of the atom is located in the central region called the nucleus 3) the nucleus consists of positively charged particles called protons and neutral particles called neutrons. 4) tint negatively charged particles called electrons travel around the nucleus in well defined paths called orbits |
ENPNEO |
|
|
Mass Number |
Corresponds to the total number of particles Present in a single atom |
|
|
|
Atomic Number |
Corresponds to the number of protons or electrons present in a single atom |
|
|
|
#neutrons= |
#neutrons=mass#-atomic# |
|
|
|
What is the charge of a proton? |
1+ |
|
|
|
What is the mass of a proton? |
1u |
|
|
|
Where are protons located? |
Nucleus |
|
|
|
What is the charge of an electron? |
1- |
|
|
|
What is the charge of an electron? |
1- |
|
|
|
What is the mass of an electron? |
1/1837u |
|
|
|
Where are electrons located? |
Orbit (orbiting the nucleus) |
|
|
|
What is the charge of a neutron? |
0 |
|
|
|
What is the mass of a neutron? |
1u |
|
|
|
Where are neutrons located? |
Nucleus |
|
|
|
Where is the mass#, atomic#, symbol, and name located in isotopic notation? |
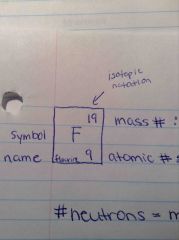
Back (Definition) |
|
|
|
Explain the relatedness of protons, electrons, neutrons, atoms, cations, anions, and molecules. |
Atoms are neutral particles composed of Protons, electrons, and neutrons(particles). If these atoms gain electrons, they become negatively charged and are called cations. If they loose electrons, they become positively charged and are called anions. When atoms join together, they create molecules. |
|

