![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
110 Cards in this Set
- Front
- Back
|
How many carbon atoms exist in 12amu of 12C?
|
By definition, there are 12amu in 1 atom of 12C. An atomic mass unit or amu is one twelfth of the mass of an unbound atom of carbon-12.
|
|
|
Which of the following most likely represents the correct order of ion size from greatest to smallest?
A) O2-, F-, NA+, Mg2+ B) Mg2+, NA+, F-, O2- C) Na+, Mg2+, O2-, F- D) Mg2+, Na+, O2-, F- |
This is an isoelectric series, which means that the number of electrons on each ion is the same. In an isoelectric series of ions, the nuclear charge increases with increasing atomic number and draws the electrons inward with a greater force. The ion with the fewest protons produces the weakest attractive force on the electrons and thus has the larger size. The answer is A.
|
|
|
If you are asked a question about finding the empirical formula of a compound and you are given the percentages of each atom by mass, what is the first thing that you do?
|
First and foremost, assume that the total number of grams of the sample is 100g. Then, multiply each percentage by 100g to get the number of grams of each atom. Then divide those grams by the atom's molecular weight to get the number of moles of each atom. Then, divide the moles of each of the atoms by moles of the atom with the smallest number of moles. This will give the molar ratio of each compound for the empirical formula.
|
|
|
When gaseous ammonia is passed over solid copper(II)oxide at high temperatures, nitrogen gas is formed:
2NH3(g) + 3CuO(s) yields N2 (g) + 3Cu(s) + 3H2O(g) What is the limiting reagent when 34 grams of ammonia form 26 grams of nitrogen in a reaction that runs to completion? |
Since the number of moles of NH3 in the equation is 2 and the number of moles of CuO in the equation is 3, we need to figure out a way to compare the two equally. We will use the number 6 because it is the next number that both divide into evenly. If we have 6 moles of NH3, 3 moles of N2 gas will be formed. If we have 6 moles of CuO, 2 moles of N2 gas will be formed. Since there will be less N2 gas formed from CuO, CuO is the limiting reagent.
|
|
|
If the position of an electron is known with 100% certainty, which of the following cannot be determined for the same electron?
A) mass B) velocity C) charge D) spin quantum number |
According to the Heisenberg uncertainty principle, both the position and the momentum (mv) of an electron cannot be known with absolute certainty at the same time. Since we know the mass of an electron, the uncertainty must lie in the velocity. The more precisely one property is known, the less precisely the other can be known. According to Heisenberg its meaning is that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle with any great degree of accuracy or certainty.
|
|
|
What is another name for a group in a periodic table?
|
Family
|
|
|
At moderately high pressures, the PV/RT ratio for one mole of methane is less than one. What must the deviation be due to?
|
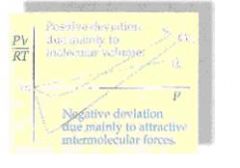
An ideal gas has PV/RT equal to one. Real volume is greater than predicted by the ideal gas law, and real pressure is less than predicted by the ideal gas law. Volume deviations are due to the volume of the molecules, and pressure deviations are due to the intermolecular forces. Thus, a negative deviation in this ratio would indicate that the intermolecular forces are having a greater effect on the nonideal behavior than the volume of the molecules.
|
|
|
A force is applied to a container of gas reducing its volume by half. Does the temperature of the gas increase, decrease, or remain constant?
|
The force does work on the gas, which means that the internal energy of the gas is increased. Since the internal energy of the gas is increased, and the numbe rof moles remains the same, the temperature, which is average kinetic energy per mole, also increases. It doesn't have to do with PV = nRT because force has added energy to the system.
|
|

The table shows 3 trials where the initial rate was measured for the reaction:
2A + B yields C What is the correct rate law for the reaction? |
When the concentration of B is doubled, the rate doesn't change. When the concentration of A is doubled, the rate doubles. The reaction is first order overall, the first order with respect to A. By choosing a trial, such as trial 2 and setting the rate 5x10^-3 = constant(5x10^-2), we find that the rate constant is 0.1. So the rate law is rate = 0.1[A].
|
|
|
As temperature is increased in an exothermic gaseous reaction, all of the following increase except:
A) reaction rate B) rate constant C) activation energy D) rms molecular velocity |
Exothermicity concerns the thermodynamics of the reaction, and not the rate. You can ignore it for the purposes of this question. The energy of activation is the energy required for a collision of property oriented molecules to produce a reaction. This does not change with temperature. Root mean square velocity (rms): Its is the square root of the mean of the squares of the velocity of a large number of molecules of the same gas. Kinetic energy is related to velocity and increases with temperature.
|
|
|
What kind of half life do first order reactions have? How do you determine how much a substance should be produced over time?
|
A first order reaction has a constant half-life. If you are given a substance Compound X that turns into Compound Y at time = 0 and time = 15min, count the amount of compound Y that has been created at the first 15 minutes. The first 15 minutes would be one half life. The next 15 minutes, would be the next half life, so half of the remaining Compound X should turn into Compound Y. The reaction is proportional to the concentration of the compound in the rate law.
|
|
|
When a radioactive isotope undergoes nuclear decay, the concentration of the isotope decreases exponentially with a constant half life. What kind of reaction is the radioactive decay?
A) zeroth order reaction B) first order reaction C) second order reaction D) third order reaction |
The concentration of reactants decreases exponentially in a first order reaction. Another way of saying is that the graph of the reactants will be linear. Also, first order reactions have a constant half life.
|
|
|
All of the following are true concerning a reaction at equilibrium except:
A) the rate of the forward reaction equals the rate of the reverse reaction B) There is no change in the concentrations of both the products and the reactants C) The activation energy has reached zero D) The Gibbs free energy has reached a minimum |
The activation energy is dictated by the reaction itself and doesn't change during the reaction. Gibbs free energy is at a minimum when a reaction is at equilibrium.
|
|
|
For a reaction that begins with only reactants and moves to equilibrium, does the rate of the the forward and reverse reactions increase and/or decrease?
|
Initially, there are no products, so the reverse reaction begins at zero. It begins to increase. As the reactants are used up, the forward reaction slows down. Equilibrium is the point where the rates equalize.
|
|
|
Calcium carbonate decomposes to calcium oxide and carbon dioxide gas via the following reversible reaction:
CaCO3(s) yields CaO(s) + CO2(g) Beaker I contains pure CaCO3 and Beaker II contains pure CaO. The container is sealed. The partial pressure equilibrium constant for the decomposition of CaCO3 is Kp. If Beaker II is removed, when would equilibrium not be achieved based on if Kp is less than, greater than, or equal to the partial pressure of CO2? |
The equilibrium expression for this equation is: Kp is equal to the partial pressure of CO2. If Kp were less than the partial pressure of CO2, then the reaction would want to go to the left, but there would be no CaO to react to form CaCO3. If Kp is greater than the partial pressure of CO2, then the reaction would want to go to the right. Equilibrium could be achieved if it went to the right because CaCO3 alone can produce CaO and CO2, but CO2 cannot produce CaCO3 without CaO.
|
|
|
Three blocks made from the same insulating material are placed between hot and cold reservoirs. Block X is the shortest and closest to the hot reservoir. Block Y is the medium size and is the the middle. Block Z is the longest and closest to the cold reservoir. What is the temperature difference between Block X and Block Z and the rate of heat flow?
|
The temperature difference is directly proportional to the distance between two points of the same material. The short blocks have less of a temperature difference than the longer blocks. The rate of heat flow is constant throughout the blocks, else heat would build up at the point of slowest flow. Because heat flow rate is constant, changing the order of the blocks won't change the rate of heat flow.
|
|
|
A box sliding down an incline increases in temperature due to friction. What type of energy transfer is taking place? Is there heat involved?
|
Unless the box and the incline are at different temperatures, there can be no heat. Energy transfer due to friction is work.
|
|
|
Which of the following gas properties is needed to calculate the work done by an expanding gas?
I) The initial and final pressures II) The initial and final volumes III) The path followed during the expansion |
All gas properties are needed. Work is not a state function, thus we must know the path in order to calculate it. Work is a path function, a different pathway results in a different amount of work.
|
|
|
What is thermal conductivity?
|
The rate at which heat conducted is directly proportional to the difference in temperatures between the hot and cold reservoirs. The bigger the difference in temperature, the greater the rate of heat conduction. In conduction, higher energy molecules of one system transfer some of their energy to lower energy molecules of the other system via molecular collisions. Direct physical contact is required. The larger the difference of temperature or the smaller the length, the bigger the rate of heat flow.
|
|
|
Which of the following properties of a gaseous system affect its enthalpy?
I) Pressure II) Volume III) Internal energy |
All affect enthalpy. The definition of enthalpy is: H = E + PV. E is internal energy
|
|
|
The heats of combustion for graphite and diamond are as follows: C of graphite(s) + O2(g) yields CO2(g) ∆H = -394kJ
C of diamond(s) + O2(g) yields CO2(g) ∆H = - 396kJ Diamond spontaneously changes to graphite. What is the change in enthalpy accompanying the conversion of two moles of diamond to graphite? |
-4kJ. This is Hess’s law. We reverse the equation for graphite, so that graphite is the product. In doing so, we must also reverse the sign of the enthalpy. Now we add the two equations and their enthalpies. Don’t forget that we must multiply by two for the two moles. We do this because the overall reaction accounts for 1 mole of diamonds as the reactants, but we are working with 2 moles of diamonds. The overall reaction is 1 mole of C diamond yields 1 mole of C graphite. If we want 2 moles of diamonds, then we would multiply the overall H by 2 to account for it. In actuality, 2 moles of graphite would also be made. So, -396kJ + (+394)kJ = -2kJ. Then we multiply -2kJ by 2 for the 2 moles, we get -4kJ. Hess’s law says that when you add reactions, you can add their enthalpies.
|
|
|
The standard enthalpy of formation for liquid water is:
H2(g) + O2(g) yields H2O(l) ∆H = - 285.8kJ/mol Which of the following could be the standard enthalpy of formation for water vapor? A) -480.7kJ/mol B) -285.8kJ C) -241.8 kJ/mol D) +224.6 kJ/mol |
-241.8 kJ/mol. Condensation must occur to form liquid water. Condensation is an exothermic process, so the formation of liquid water should be more exothermic than the formation of water vapor. The standard enthalpy of formation of water vapor will not be an endothermic process, so D is wrong. It’s not completely endothermic because there is still water involved…it’s a balance between water and the gas above it.
|
|
|
Which of the following is a violation of the law of conservation of energy?
A) Heat can be changed completely to work in cyclical process B) A system undergoing a reaction with constant enthalpy experiences a temperature change C) After sliding to a stop, a boxy with initial kinetic energy K has only thermal energy in an amount less than K D) A bond is broken and energy is released |
D. Energy is always required to break a bond. The answer is not C because some energy is lost to friction.
|
|
|
The reaction below shows the condensation of water:
H2O(g) yields H2O(l) Which of the following will be positive for water at 25 degrees Celsius and 1 atm? A) ∆H B) ∆S C) ∆G D) None of the above |
Bonds are formed when water condenses, so energy is released and ∆H is negative. The water molecules become less random, so ∆S is negative. ∆S is positive when molecules are more random (such as gas molecules). Condensation occurs spontaneously at 25 degrees Celsius (room temperature), so ∆G is negative.
|
|
|
The normal boiling point of benzene (C6H6) is 80.1 degrees Celsius. If the partial pressure of benzene gas is 1 atm, which of the following is true of the reaction shown below at 80.1 degrees Celsius?
C6H6(l) yields C6H6(g) A) ∆S is negative B) ∆S is zero C) ∆G is negative D) ∆G is zero |
∆D is zero. At the boiling point, benzene is in equilibrium between the liquid and gas phases. At equilibrium, ∆G for a reaction is equal to zero. S is positive for the reaction shown because gases are more random than liquids.
|
|
|
What is the approximate molarity of a NaCl solution with a specific gravity of 1.006?
|
Learn this: One liter of water has a mass of 1kg. One liter of this solution weighs 1.006 kilograms, because it's specific gravity is 1.006kg/L. Specific gravity is the heaviness of a substance compared to that of water, and it is expressed without units. If something is about 7.85 times as heavy as an equal volume of water (such as iron is) its specific gravity is 7.85. Its density is 7.85 grams per cubic centimeter, or 7.85 kilograms per liter, or 7.85 metric tons per cubic meter.
Since the gram was defined as the mass of a cubic centimeter of water, water must by definition have a density of 1 gram per cubic centimeter. If we assume that the volume of water changes very little when NaCl is added, then about 0.006kg or 6g of NaCl are in each liter of solution. The molecular weight of NaCl is 58.6 g/mol. 6 grams is about 0.1 moles. |
|
|
Which of the following substances is least soluble in water?
A) NH3 B) NaCl C)HSO4- D)CCl4 |
The answer is D. Remember that like dissolves like. Water is polar, and will dissolve polar and ionic substances. A, B, and C are ions, ionic compounds, or capable of hydrogen bonding. Carbon tetrachloride is a nonpolar molecule.
|
|
|
A solution contains 19g of MgCl2 in 0.5L of distilled water. If MgCl2 totally dissociates, what is the concentration of chloride ions in the solution?
|
First calculate the number of moles of MgCl2. Moles = grams/MW = 19g/95g/mol = 0.2 moles. We are looking for the concentration of chloride ions, so 0.2 moles of MgCl2 will dissociate to produce 0.4 moles of Cl- ions because there are twice as many Cl- ions as there are MgCl2 molecules. Cl- = moles/liters = 0.4 mol/0.5L = 0.8M
|
|
|
A student has 0.8 liters of a 3 molar HCl solution. How many liters of distilled water must she mix with the 3 molar solution in order to create a 1 molar HCl solution?
|
First find the number of moles of HCl. moles = (mol/L) = 3 mol/L(0.8L) = 2.4 moles
Now find the number of liters needed to make the solution 1 molar. L = mol/mol/L = 2.4 mol/1 M = 2.4 L Now be careful. The student already used 0.8 liters of solution, so in order to get 2.4 L, you have to add 1.6 L of water. |
|
|
NaCl dissolves spontaneously in waer. Based upon the following reaction:
NaCl(s) yields Na+(g) + Cl-(g) ∆H = 786 kJ/mol, the heat of hydration for NaCl must be: A) negative with a magnitude less than 786 B) negative with a magnitude greater than 786 C) positive with a magnitude greater than 786 D) Nothing can be determined about the heat of hydration without more information |
The answer is D. The change in entropy is positive in solution formation and Gibbs free energy is negative in a spontaneous reaction. From ∆G = ∆H - T∆S, we see that the heat of solution may be either positive or negative in this case. The heat of hydration is the separation of water molecules (which requires energy) and the formation of bonds between the ions and water molecules (which releases energy). Thus, the value of the heat of hydration could be either negative or positive.
|
|
|
Which of the following indicates an exothermic heat of solution?
A) Heat is evolved B) The final solution is acidic C) A precipitate is formed D) The reaction is spontaneous |
The answer is A. At constant pressure, change in enthalpy is equal to change in heat. Heat is evolved in exothermic processes and absorbed in endothermic processes. Under conditions of constant pressure (e.g. most biological processes under constant atmospheric pressure) the heat absorbed or released is termed enthalpy (or "heat content"). In formal terms: The change in enthalpy, ∆H, equals the heat, q, added to or lost by the system when the process occurs under constant pressure: ∆H=q. ∆H represents the difference between the enthalpy of the system at the beginning of the reaction compared to what it is at the end of the reaction:
∆H= Hfinal - Hinitial |
|
|
When two pure liquids, A and B, are mixed, the temperature of the solution increases. Is the vapor pressure of the solution greater than, less than, or equal to both the vapor pressure of pure A and pure B?
|
The vapor pressure of solution might be lower than just one of the pure substances but no the other. Raults's law says that the vapor pressure of the solution is equal to the mole fraction times the vapor pressure of each substance added together.
|
|
|
Which of the following will increase the vapor pressure of a liquid?
A) Increasing the surface area of the liquid by pouring it into a wider container B) increasing the kinetic energy of the molecules of the liquid C) decreasing the temperature of the liquid D) adding a nonvolatile solute |
Molecules break free of the surface of a liquid and add to the vapor pressure when they have sufficient kinetic energy to break the intermolecular bonds. Vapor pressure also increases with temperature. Vaporization is an endothermic process.
|
|
|
When two volatile solvents are mixed, the vapor pressure drops below the vapor pressure of either solvent in its pure form. What is the heat of the solution?
|
Volatile solvents (or solutes) are solvents with a vapor pressure. Nonvolatile solvents (or solutes) are solvents with no vapor pressure. The heat of the solution is the overall change in energy of the reaction and it is equal to the change in enthalpy. It is ∆H of the solution. So a negative heat of solution results in stronger intermolecular bonds and gives off heat, while a positive heat of solution results in weaker intermolecular bonds and it absorbs heat. Negative heats of solution also lower vapor pressure and positive heats of solution raise vapor pressure
|
|
|
A solution composed of ethanol and methanool can be thought of as ideal. At room temperature, the vapor pressure of ethanol is 45 mmHg and the vapor pressure of methanol is 95 mmHg. Predict what the vapor pressure of a solution will be if it contains only ethanol and methanol?
|
It will be greater than 45 mmHg and less than 95 mmHg. In an ideal solution, the vapor pressure will be somewhere in between the vapor pressures of the solute and the solvent, depending on their relative mole fractions.
|
|
|
What is Raoult's law for nonvolatile solutes?
|
When a nonvolatile solute (a solute with no vapor pressure) is added to a liquid, some of those solute molecules will reach the surface of the solution and reduce the amount of surface area available for the liquid molecules. Since the solute molecules don't break free of the solution but do take up surface area, the number of molecules breaking free from the liquid is decreased while the surface area of the solution and the volume of open space above the solution remain the same. Here, the vapor pressure of the solution is equal to the mole fraction of the liquid times the vapor pressure of the pure liquid.
|
|
|
What is Raoult's law for volatile solutes?
|
If the solute is a volatile solute (a solute with a vapor pressure), the situation is a little more complicated. A volatile solute will also compete for the surface area of a liquid. However, some of the molecules of a volatile solute will escape from solution and contribute to the vapor pressure. If the solution is an ideal solution (solute and solvent have similar properties), the partial pressures contributed by the solvent and solute can be found by applying Raoult's law separately. So the total vapor pressure of the solution is equal to the sum of the partial pressures of the solvents. The partial pressure is the mole fraction of the solvent times the vapor pressure of the solvent.
|
|
|
When a solution is saturated:
A) the solvent changes to solute, and the solute changes to solvent at an equal rate B) the vapor pressure of the solution is equal to atmospheric pressure C) the concentration of solvent is at a maximum D) the concentration of solvent is at a minimum |
D. is correct.Think in terms of mole fraction. The concentration of solvent is at a minimum when the concentration of solute is at a maximum. There is more solute than solvent, so much so that the solute is at a maximum level, so in turn, there's a minimum level of solvent.
|
|
|
Na2SO4 dissociates completely in water. If Na2SO4 were added to a solution containing equal concentrations of aqueous Ca2+, Ag+, Pb2+, and Ba2+ ions, what will determine which compounds (CaSO4, Ag2SO4, PbSO4, and BaSO4) will precipitate first?
|
This is the common ion effect. The solid that would precipitate first would be the solid that has the lowest Ksp - solubility product constant. This is because the concentration of the reactants is larger than the concentration of the products.
|
|
|
The Ksp of BaCO3 is 1.6x10^-8. How many moles of barium carbonate can be dissolved in 3 liters of water?
|

This is the saturated concentration in mol/L. We multiply this by 3 liters to get the number of moles. So the answer is 1.2 x 10^-4.
|
|
|
If the solubility of PbCl2 is equal to x, what is the solubility product for PbCl2 in terms of x?
|
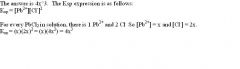
|
|
|
20 grams of NaCl is poured into a coffee cup calorimeter containing 250 ml of water. If the temperature inside the calorimeter drops 1 degrees Celcius by the time the NaCl is totally dissolved, what is the heat of solution for NaCl and water? (specific heat of water is 4.18 J/(g * degrees Celsius))
|
First figure out the heat evolved by the reaction using q = mc∆T. q = 250 grams * (4.18J/g(*degrees celsius ))* (1 degrees celsius)
Next divide by moles of NaCl (20 grams is about 1/3 of a mole because 20/60 is about 1/3). This givies you 3150 joules, which is equal to 3kJ. Since the temperature went down, the reaction is endothermic with positive enthalpy. Round everything. |
|
|
How does specific heat vary with different phases of the same substance? How does temperature change in a substance if heat is added and the substance is allowed to expand?
|
Different phases will have different specific heats. When heat is added to a fluid, its temperature will change less if it is allowed to expand.
|
|
|
Why are boiling points a better indication of intermolecular bonding than melting points?
|
Transition from solid to liquid involves other factors such as crystalline lattice structures. Crystallization depends upon molecular symmetry as well as intermolecular bonding. Boiling point is strongly dependent upon intermolecular bond strength.
|
|
|
A reaction occurs in absorption chambers in which Chamber 1 absorbs all of the H2O and Chamber 2 absorbs all of the CO2. Welding gas, which is only made of C and H atoms is involved in a combusion. It is similar to the propane combustion of C3H8 + 5O2 yields 4H2O + 3CO2. The mass of the absorbers in Chamber 1 increases by 0.9 grams and the mass of absorbers in Chamber 2 increases by 4.4 grams. What is the empirical formula for welding gas?
|
Only water is caught in chamber 1. The change in mass of chamber 1, 0.9 grams, is all water. 0.9 grams of water divided by 18g/mol gives 0.5 mole of water. All of the hydrogen came from the sample, and all the oxygen came from excess oxygen. Because the hydrogen is from the sample, for every mole of water, there are 2 moles of hydrogens, so there is 0.05 x 2 = 0.1 mole of hydrogen in the sample. Doing the same with the carbon dioxide caught in chamber 2 we have: 4.4/44.2 = 0.1 of CO2, or 0.1 mole of carbon from the sample. We don't care about the oxygen because the oxygen is reacted with the sample. This is a 1:1 ratio. The empirical formula is CH. If oxygen was in the welding gas, we would not be able to compare the moles of hydrogen to water in this same way.
|
|
|
The density of welding gas with an empirical formula of CH is 1.1 g/L at 25 degrees Celsius and atmospheric pressure. At the same conditions, O2 has a density of 1.3 g/L. What is the molecular weight of welding gas?
|
The molarity of O2 is equal to the molarity of the welding gas or any other ideal gas at the same temperature and pressure (Avogadro's Law of ideal gases). Density divided by molecular weight is molarity. Molarity is moles/L and it is equal to g/L (density) * mol/g (molecular weight divided into density). Therefore, we can set the ratios of density to molecular weight for oxygen and the welding gas equal to each other. We get: 1.3/32 = 1.1/M.W. The molecular weight is 26 g/mol.
|
|
|
What is PV/nRT equal to?
|
PV/nRT is equal to 1. This is always for an ideal gas.
|
|
|
هَدَفَ - يَهدُف ل أو إلى
|
to aim to
|
|
|
A heterogenous catalyst is a catalyst that involves the reactant molecule bonding to the catalyst for the reaction to occur. The rate of a reaction may depend on which of the following:
I) [Reactants] II) [catalyst] III) Surface area of the heterogeneous catalyst IV) Temperature |
The rate of a reaction may depend on all 4 things. Standard rate law tells you that the rate is dependent on the concentration of the reactions. The ratio of concentration of the catalyst and the substrates affect the rate of a ration. This rate can be changed by changing the concentration of the catalyst. This is saturation kinetics exhibited by enzyme catalysts. Thus, the [catalyst] can affect the rate of a reaction. Increasing the surface area of a heterogenenous catalyst is like increasing the concentration of it.
|
|
|
H2 can be added to ethylene in the presence of a heterogenous catalyst such as solid platinum. What might account for the initial attraction between the hydrogen molecules and the solid platinum?
|
Van der Waals attraction. Van der Waals forces is a synonym for London Dispersion Forces. They are intermolecular forces. Hydrogen bonding requires a hydrogen atom bonded to a nitrogen, fluorine, or oxygen only.
|
|
|
What is the relationship of the rate of the slow step of the equation to the rate of the overall reaction?
|
The rate of the slow step is equal to the rate of the overall reaction.
|
|
|
The rate expression for the reaction of H2 with Br2 is:
rate = k[H2][Br2] What is the rate order with respect to H2 and the overall rate order? |
The rate is first order with respect to H2 and second order overall. The exponents in the rate law indicate the order of the reaction with respect to each concentration.
|
|
|
Which of the following are true concerning any reaction at equilibrium?
I) [products] = [reactants] II) The rate of ∆[products] = rate of ∆[reactants] III) The rate constant of the forward reaction is equal to the rate constant of the reverse reaction |
Only II. In a reaction at equilibrium, the rate of change in the [products] and the [reactants] is zero. This does not mean that the [reactants] and [products] are equal, nor that the rate constants are equal. As reactants are converted to products, the [reactants] decreases and the [products] increases. Since rates are related to concentrations, the rate of the forward reaction begins to slow and th rate of the reverse reaction quickens as a reaction proceeds. Eventually, the two rates become equal. When the forward reaction rate equals the reverse reaction rate is when equilibrium occurs. The net reaction rate is zero, but there is a forward and reverse reaction rate. The rate constant k is not necessarily equal. At equilibrium, there is no change in the [products] or [reactants]. Equilibrium is a dynamic process.
|
|
|
Ni(s) + 4CO(g) yields Ni(CO)4(g)
standard ∆H = -160.8 kJ What is the standard ∆H of formation of solid Nickel? |
The standard ∆H of formation of solid Nickel is 0. Nickel is an element and is a solid in its natural state at 298 K (25 degrees Celsius, standard conditions). Thus, the enthalpy of formation of solid nickel at 298K is zero.
|
|
|
Does the entropy of the system have to be positive in order for a reaction to be spontaneous?
|
A reaction can be the system and everything outside the reaction can make up the surroundings. The entropy change in a system does not have to refer to the universe. The second law of thermodynamics says that a reaction is spontaneous when the entropy of the universe, not the system, is positive. The entropy of the system may be positive or negative.
|
|
|
A certain Carnot engine requires 18 kg of water in the form of steam as its working substance. When 5 x 10^5 J of heat energy are added at a contant temperature of 400K, the gas expands to 4 m^3. What is the approximate pressure of the gas after initial expansion? (The ideal gas constant is R = 8.314 J/K mol)
|
The answer is 8.3 x 10^5 Pa. PV = nRT. Make sure to convert 18 kg into g/mol. By the way, if the gas did not behave ideally, the real pressure would be lower. In this case, the gas does behave very ideally because it is at high temperature.
|
|
|
What determines whether a reaction is the most thermodynamically favored and the most kinetically favored?
|
The smallest energy of activation is the most kinetically favored. The largest drop in energy is the most thermodynamically favored.
|
|
|
What is the change in energy of a reaction?
|
The change in energy is the energy of the products minus the energy of the reactants. This does not include the activation energy.
|
|
|
A metal rod is in the thermal contact with two heat reservoirs both at constant temperature, one at 100K and the other at 200K. The rod conducts 1000J of heat from the warmer to the colder reservoir. If no energy is exhanged with the surroundings, what is the total change of entropy?
|
The entropy of the system is equal to the change in entropy of the two reservoirs. ∆S = Q/T for each reservoir. Q is heat energy in Joules, T is temperature in Kelvins. The change in entropy of the first reservoir is negative because heat energy is leaving the system (-1000/200 = -5), and the change in entropy of the second reservoir is positive because heat energy is entering the system (1000/100 = 10). The sum of the two entropy changes is +5 J/K. The change in entropy for any isolated system must be positive for any irreversible process. It is equal to 0 for a reversible process.
|
|
|
Two ideal gases, A and B, are at the same temperature, volume, and pressure. Gas A is reversibly expanded at constant temperature to a volume V. Gas B is allowed to expand into an evacuated chamber until it also has a total volume V, but without exchanging heat with its surroundings. How do the temperatures and enthalpies for these gases compare to each other?
|
Gas A and B have equal temperatures and enthalpies. The temperature of Gas A remains constant because the questions says so. Temperature is kinetic energy (due to random mostion) per mole. Gas B does no work and doesn't exhcange heat so its energy doesn't change; it has the same kinetic energy (due to random motion) per mole as when it began. Thus, its temperature doesn't change either. Enthalpy is PV + U. P and V are the same for both gases because they are temperature, volume, and therefore pressure (PV = nRT). U doesn't change for Gas A because any energy removed is replaced to keep the temperature the same because there is no change in temperature. U doesn't change for Gas B because no energy is exchanged with the surroundings for Gas B.
|
|
|
How do you calculate equations that are raised to fractions? Such as, what is 2 ^3/2 = x
|
If you square both sides, you get 2^3 = x^2 = 8. Then you square root both sides to get x.
|
|
|
A 25 mL sample of hard water with Ca2+ and Mg2+ ions is titrated with a 0.001 M solution of EDTA, and the endpoint of the titration is reached at 50 mL of EDTA added. What is the concentration of Ca2+ and Mg2+ ions in solution? There is 1 to 1 stoichiometry between EDTA and the ions.
|
Basic Rules for Solving Titration Problems. (1)Three of the four values must be known. (2)The units of concentration MUST be mol/L. (3)The units of volume and concentration must be the same respectively.(4) It is crucial that the volume and concentration that relate to one anotherare identified. Use the equation: c1v1 = c2v2, where c1 and c2 are the concentrations of the titrant and solution, and v1 and v2 are the volumes of the titrant and solution. (50mL)(0.001M) = (25 mL) (x). x = 0.002 M.
|
|
|
9 ppm is equivalent to an aqueous concentration of approximately 5 x 10^-4 mol/L. If a water sample were reduced from 18 ppm Mg2+ to 9 ppm Mg 2+ by the addition of EDTA, what would be the concentration of the remaining unbound EDTA?
EDTA + Mg2+ yields EDTA-Mg The association constant K = 5 x 10^8. |
The association constant k = 5 x 10 ^8 = [EDTA-Mg]/[EDTA][Mg2+]
Since half the magnesium is bound, [EDTA-Mg] is 9ppm, which is 5 x 10^-4. The remaining half of [Mg2+] is 9 ppm, which is also 5 x 10^-4. Plugging these into the equilibrium expression leaves the remaining concentration [EDTA] = 1/(5 x 10^8). |
|
|
Which salt is the most efficient per gram at lowering the freezing point of water?
A) Ba(OH)2 B) MgSO4 C) NaCl D) CaCl2 |
The answer is NaCl. Even if the others completely dissociate, NaCl still releases more particles per gram than the others. NaCl releases 2 particles/58 grams. Ba(OH)2 releases 3/172, MgSO4 releases 2/120, and CaCl2 releases 3/115.
|
|
|
A solid was dissolved into a liquid in a test tube. The test tube and its contents were lowered into an ice bath. The temperature of the solution was monitored and readings were made intermittently. A stirrer was used in the test tube. What is the purpose of the stirrer?
|
The purpose of the stirrer is to ensure that the solution temperature remains homogenous. The stirrer acts to evenly distribute the heat throughout the solution by convection. Convection is the transfer of heat by the actual movement of the warmed matter. Convection is the transfer of heat energy in a gas or liquid by movement of currents. The heat moves with the fluid. Consider this: convection is responsible for making macaroni rise and fall in a pot of heated water. The warmer portions of the water are less dense and therefore, they rise. Meanwhile, the cooler portions of the water fall because they are denser.
|
|
|
0.500 gram of an unknown solid was dissolved into a solution. Student 1 had results of the solution having a lower freezing point that Student 2. Which student had the unknown with the greatest molecular weight?
|
Student 2. Since the freezing point depression was lower for Student 1, there must have been more particles for the same amount of mass. Thus, Student 1 had an unknown with lower molecular weight, because molecular weight is g/mol. Student 1 had a greater number of moles than Student 2. Moles and particles are the same thing.
|
|
|
10.00 mL of cyclohexane is placed into a test tube at room temperature. Then, 0.500 gram of an unknown solid is dissolved in the cyclohexane. The test tube and solution is lowered in an ice bath and the temperature of the solution is read intermittently. A professor must choose the unknown from the following solutes. Which would be most appropriate?
A) NaCl B) Mg(OH)2 C) CH3OH D) C10H8 |
Answer D, C10H8 would be most appropriate. D is the only nonvolatile, nonpolar solute that is soluble in cyclohexan.
|
|
|
On his honeymoon, the chemist, Joule, took with him a long thermometer with which to measure the temperature difference between the waters at the top and bottom of Niagara Falls. If the height of the falls is 60 meters and the specific heat of water is about 4200 J/(kg*K), what is the expected temperature difference?
|
The potential energy of the water at the top of the falls becomes kinetic energy as it drops, then thermal energy at the bottom of the falls. Thus, mgh = Q = mc ∆T.
Heat capacity is C in the equation is q = C ∆T where q is in Joules, C is in J/K or cal/degrees celcius and ∆T is in K. Specific heat capacity is q = mc ∆T, where m is for mass and specific heat is J/(kg*K) or cal/(g*degrees Celsius). Heat capacity is upper case C and specific heat capacity is lowercase c. ∆T = gh/c, so the answer is 1/7K. |
|
|
Which of the following is ordered correctly in terms of atomic radius, from smallest to largest?
A) Al3+, Al, S, S2- B) Al3+, S, Al, S2- C) S, Al3+, S2-, Al D) S, S2-, Al3+, Al |
The answer is B. Positive ions are much smaller than their neutral counterparts; negative ions are much larger. Since the size of the neutral atoms decreases as you move from left to right across the periodic table, neutral aluminum is bigger than neutral sulfur. The cation would be the smallest and the anion would be the largest.
|
|
|
Removing an electron from which of the following would require the most energy?
A) Na- B) Na C) Na+ D) Na2+ |
The answer is D. As electrons are removed from an atom, each successive electron becomes harder to remove.
|
|
|
An atom of phosphorus will be most similar in size to which of the following atoms?
A) O B) Ge C) As D) Se |
Since atoms get smaller as you go to the right across the periodic table, but get larger as you go down, the size is not likely to change much as you go down and to the right (or up and to the left). This "diagonal relationship" is just a good estimate.
|
|
|
A naturally-occurring sample of which of the following has the smallest density at room temperature?
A) Beryllium B) Boron C) Fluorine D) Lithium |
The answer is C. Fluorine's a gas, and th others are solid, so a gas is less dense than a solid. Be, and Li are metals and B is a metalloid, while Fluorine is a non-metal (gas). At room temperature and pressure, fluorine and chlorine are gases, bromine is a liquid and iodine and astatine are solids; The halogen group is therefore the only group that exhibits all three states of matter at room temperature.
|
|
|
A naturally-occurring sample of which of the following has the smallest density at room temperature?
A) Carbon B) fluorine C) Nitrogen D) Oxygen |
Throw out the carbon because its a solid. The volume of an atom doesn't matter for a gas (gases are mostly empty space between atoms), but the mass does. The answer is C.
|
|
|
A naturally-occurring sample of which of the following has the smallest density at room temperature?
A) Argon B) Chlorine C) Phosphorus D) Sulfur |
Phosphorus and sulfur are solids, so throw them out. Chlorine exists as a diatomic gas, so it has a molar mass of 71 g/mol, while argon exists as a monoatomic gas, so it has a molar mass of 40 g/mol.
|
|
|
Why are two atoms held together by a chemical bond?
|
Because of the attraction of their nuclei to the bonding electrons. The force holding the electrons together is electrostatic.
|
|
|
What is the distance between two nuclei in a chemical bond determined by?
|
A balance between the repulsion of the nuclei for each other and the attraction of the nuclei for the bonding electrons. The positively charged nuclei repel each other, while they attract the bonding electrons. An equilibrium is established at the bond length.
|
|
|
How does the energy of a typical carbon-carbon double bond compare to the energy of a typical carbon-carbon single bond?
|
The bond energy of the double bond is greater than that of the single bond, but less than twice as great. A double bond comprises a sigma bond and a pi bond, while a single bond has only a sigma bond. Pi bonds are weaker than sigma bonds, but of course are better than nothing. Thus a double bond (sigma and pi) is stronger than a single bond (sigma only) but less strong than two single bonds (sigma and sigma).
|
|
|
What are the bond length in CS3 with a charge of 2- like?
|
All of the bond lengths are the same. Resonance spreads the double bond character equally among all three C-S bonds.
|
|
|
Which of the following is true of all pure compounds?
A) They are each made from a single element B) They exist as a collection of separate and identical molecules C) The relative # of atoms of 1 element compared to another can always be represented by a ratio of 2 whole numbers D) They are held together by intermolecular bonds |
The answer is C. The question is asking about compounds. All ionic compounds violate A, B, and D.
|
|
|
What is the mass of 1 molecular of water?
|
18 amu. This is essentially a question about units. Grams and moles are macroscopic units, meaning they are appropriate for an amount of material you could measure out physically. Only amu is an atomic-scale unit. If the mass of a single molecule of water were measured in grams, the number would be very small (like 10^-22).
|
|
|
Work backwards with the below mass percentage question:
The mass percent of a compound is as follows: 43.64% P and 56.36% O. What is the empirical formula? A) PO B) PO2 C) P2O3 D) P2O5 |
Choice A has an oxygen fraction of 16/(16+31), or about 30%, too low. Choice B has an oxygen fraction of 32/(32+31), which is close to 50%, just too low. Choice C is 48/(48+62), which is too low. Choice D is 80/(80+62), which is above 50% so must be the correct answer. To save time, calculate one element at a time for each choice. It's better to work backwards with percent mass and molecular weight questions because at least we know one of the answers is correct instead of trying to figure out the question on your own and seeing that your answer is not listed!
|
|
|
How can you use the answers to answer the the following question:
The mass percent of a compound is as follows: 71.65% Cl, 24.27% C; and 4.07% H. If the molecular weight of the compound is 98.96, what is the molecular formula of the compound? A) ClC2H2 B) ClCH2 C) Cl2C2H4 D) Cl3C3H8 |
We know the molecular weight is approximately 99, and we can determine the molecular weights of the answer choices easily. Unlike percent mass, there is no division (just addition). Choices A and B are too light, Choice C looks about right (2 x 35.5 + 2 x 12 + 4 x 1). We're using the process of elimination, so it doesn't have to be exact. This is a better way to figure out the answer because the molecular sample is not the assumed 100g and we would have to multiply .4364 by 99 grams, and .5636 by 99 grams to get the answer.
|
|
|
What is the empirical formula of acetic acid, CH3COOH?
A) CH4 B) CH2O C) C2H4O2 D) CH3COOH |
The answer is B. If you see atoms repeated in a compound, first gather together atoms of the same element: there are two carbons, 4 hydrogens, and 2 oxygens. Now reduce, we can divide by 2 and get the answer. Don't depend on the molecular formula to gather the same atoms together.
|
|
|
The empirical formula of a hydrocarbon is known to be CH2. Can the percent mass and/or the molar mass be determined from this information?
|
The molar mass cannot be determined from the empirical formula, since the molecular formula is known (it might, for example, be C5H10). The percent composition can be found, however, since this only depends on the relative amounts of the elements present. This is exactly the information the empirical formula provides.
|
|
|
Consider the balanced equation:
4Fe + 3O2 yields 2FeO3 If 2 moles of Fe react to completion with 2 moles of O2, what remains after the equation? |
1 mole of Fe2O3 and 1/2 mole of O2. Only 3/2 moles of O2 is needed for the reaction and the reaction started with 4/2 = 2 moles of O2. 4/2 - 3/2 = 1/2 moles of O2 left.
|
|
|
In the following reaction, which is run at 600K, 4.5 moles of nitrogen gas are mixed with 11 moles of hydrogen gas:
N2 (g) + 3H2 (g) yields 2NH3 (g) The reaction produces 6 moles of ammonia. How many moles of nitrogen gas remain? A) 0 mol B) 1.5 mol C) 3.0 mol D) 4.5 mol |
According to the balanced equation, 11 moles of hydrogen gas should react with 11/3 = 3.7 moles of nitrogen gas. Since we started with 4.5 mol of nitrogen gas, we should finish with 0.8 moles, which is not a choice! So this is a trick question: the reaction must not go to completion. We are also given the moles of ammonia: six moles of ammonia require 3 moles of nitrogen, and we started with 4.5, thus 1.5 remain.
|
|
|
In the following reaction, which is run at 600K, 4.5 moles of nitrogen gas are mixed with 11 moles of hydrogen gas:
N2 (g) + 3H2 (g) yields 2NH3 (g) The reaction produces 6 moles of ammonia. What is the percent yield of ammonia? |
Hydrogen is the limiting reagent because 11 moles of H only produce 7.3 moles of NH3, while 4.5 moles of N2 produce 9 moles of N2. Since 6 moles of NH3 were produced, 6/7.3 is the percent yield, which is 87%
|
|
|
Which of the following compounds lacks ionic bonds?
A) NaCl B) NaH C) HCl D) Ca3(PO4)2 |
The answer is HCl. Ionic compounds generally consist of a metal and a nonmetal (or occasionally a polyatomic cation like ammonium). HCl is a molecular substance; indeed it is a gas at room temperature. The fact that it splits into ions when it is placed in water does not indicate definitely that it is an ionic compound in its pure form. Chlorine forms ionic and covalent bonds. An example of a compound in which chlorine forms ionic bonds is sodium chloride, NaCl(s). An example of covalent chlorine bonding is the bond between two chlorine atoms in the Cl2 molecule. Here both atoms are identical, so there is no difference in electronegativity, and the bond is totally covalent in character. An example of chlorine forming polar covalent bonds is the hydrogen chloride molecule, HCl. Here there is a small difference in electronegativity between the Cl and H atoms. This leads to an uneven electron distribution in the bond, and electrons will tend to spend more time near the more electonegative element (in the case of HCl, the chlorine atom). The polar covalent bond can be thought of as some way between a covalent bond (where bonding electrons spend equal time around both atoms in the bond), and an ionic bond (where bonding electrons spend 100% of the time around the more electronegative atom in the bond). NaH is an ionic compound and H is a nonmetal.
|
|
|
How many quantum numbers are necessary to describe a single electron in an atom?
A) 1 B) 2 C) 3 D) 4 |
The answer is D. N, l, m, and s. At all times.
|
|
|
An electron in a certain element can have energies of -2.3, -5.1, -5.3, -8.2, and -14.9 eV. -14.9 eV is the ground state of the electron and no other levels exist between -14.9 and -2.3 eV. What could be a partial list of photon energies that could be absorbed by an electron in the ground state of this atom?
|
6.9, 9.6, 9.8, 12.6, 15.0, and 16.1 eV. You have to subtract 14.9eV from all of the levels above it. The last 2 numbers are larger than the energy of the ground state, so they can also boost the electron from one level to another.
|
|
|
Which of the following must be true of an electron in the hydrogen atom?
A) Since there is only 1 electron, that electron must be in the lowest energy level B) The spacing between n=1 and n=2 energy levels is the same as the spacing between the n=4 and n=5 energy levels? C) The energy of each level can be computed from a known formula D) The energy levels are identical to the levels in the He+ ion |
C is correct. Choice D is not true because the charge on the nucleus is greater than the He+ ion.
|
|
|
In the ideal gas, what does the variable V represent?
|
The volume of the container which holds the gas. In fact, in the kinetic-molecular theory of gases, the volume of each gas molecule is assumed to be zero.
|
|
|
Which of the following affects the average force (per unit area) exerted by a gas on the wall of the container?
I. The average speed of a gas molecule II. The frequency of collisions between gas molecules and the wall III. The volume of a gas molecule |
The answer is I and II. All else being equal, a faster molecule has more momentum, and thus exerts more force on the wall. Likewise, more collisions means more force (on average). But the volume of a molecule is irrelevant to the average force. Volume does not enter into Newton's laws!
|
|
|
Is the graph of pressure and temperature a positive slope graph or a positive parabola graph?
|
It is a positive parabola.
|
|
|
Container A contains gas at 300 degrees Celsius and Container B contains the same gas, but at 150 degrees Celsius. Do all of the gas molecules in Container A move faster than all of the molecules in Container B?
|
No. In any gas, molecules have a wide range of speeds. In a gas at 300 degrees Celsius, the average speed of a molecule will be greater than a gas at 150 degrees, but the spread will be very wide in both cases.
|
|
|
Gas A is at 25 degrees Celsius and 1 atmosphere. If the pressure is increased to 3 atmospheres without changing the volume, the new temperature will most likely be:
A) -174 degrees C B) 8.3 degrees C C) 28 degrees C D) 621 degrees C |
The answer is D. According to the ideal gas law, the new temperature should be triple the value of the original. But be careful! Temperatures must be expresed in Kelvins when you're using the ideal gas law. So 25 degrees C is 298 K, which when tripled, is almost 900K, or about 600 degrees C (not 75 degrees C). Alternatively, you might realize that tripling the pressure at constant volume and number of moles should increase the temperature a lot.
|
|
|
An ideal gas with pressure 2 atmospheres is expanded to twice its initial volume. What is the new pressure?
|
This information cannot be determined from the information given. We have to know what happened to the temperature. Is it constant? Did it change?
|
|
|
Can pressure and volume be used to estimate the value of absolute zero?
|
Yes, according to the gas law, both pressure and volume are directly proportional to (absolute) temperature: PV=nRT.
|
|
|
Which of the following can be used as a half-cell in an electrochemcial cell along with Cu/Cu2+? E° (volts) = 0.34
I. Al3+ + 3e- → Al (E0 = -0.76) II. Cu+ + 2e- → Cu (E0 = 0.34) III. F2 + 2e- → 2F- (E0 = 2.87) (a) I only (b) II only (c) III only (d) I and III only |
Any half-cell can be used to create an electrochemical cell so long as they have two different cell potentials. If they have the same potential, they will create no net difference between the two of them. If any difference exists, electrons will flow, creating a current. The answer is d.
|
|
|
What is the molecular weight the compound formed by the condensation of 3 NH2CH2COOH, assuming each subunit is joined by a single bond?
(a) 171 amu (b) 189 amu (c) 207 amu (d) 225 amu |
Condensation reactions involve the joining of two compounds with the loss of water. In condensing three compounds together we form two bonds and thus lose two water molecules, each of molecular weight 18. The original subunits have a mass of 16 (NH2) + 14 (CH2) + 45 (COOH) = 75. 75 amu x 3 subunits gives 225 amu - (18 x 2) = 189
The answer is B. |
|
|
A solution of H2SO4 (aq) has a pH of 6.0. What is the concentration of H30+ (aq)?
|
For these questions, use the pH = -log[H+] equation. This will give you the concentration of H+ or H30+, but H30+ is the same as H+. 6.0 = -log[H+], so the antilog(-6.0) = [H+]. So [H+]= 1x10^-6. Just look for the answer with the negative of the pH raised to the 1x the 10th power.
|
|
|
The ability of NH3 to form coordination compounds with transition metal ions can be best accounted by what ability of the NH3?
|
The NH3 can donate electrons for the complex. The nitrogen of the NH3 has a lone pair of electrons that it can donate in the covalent bond and metal ions are electron deficient. Both electrons that make up the bond between NH3 and the metal ion come from ammonia. Ammonia is the Lewis base.
|
|
|
The set of quantum numbers that correctly describes an electron in a 3p orbital is:
A) n = 3; l = 0; ml = 0; ms = 0 B) n = 3; l = 2; ml = -2, -1, 0, 1, or 2; ms = +1/2 or -1/2 C) n = 3; l = 1; ml = -1, 0, or 1; ms = +1/2 or - 1/2 D) n = 4; l = 0; ml = -1, 0, or 1; ms = +1/2 or -1/2 E) None of the above. |
C) n = 3; l = 1; ml = -1, 0, or 1; ms = +1/2 or - 1/2
Remember that l ranges in values from 0 to n-1. Although here n=3, we are looking at p orbitals, so the only l value for p orbitals is l=1. l = 0 for s orbitals, l = 1 for p orbitals, l = 2 for d orbitals, l = 3 for f orbitals. Then take it from there to find out m ( m is from -l to +l including 0). And ms is +1/2 and -1/2. |
|
|
An aluminum atom has ____ unpaired electron(s).
A) 4 B) 1 C) 7 D) 0 E) 3 |
The answer is B. Think about Hund's rule. The s orbital has 2 electrons paired, with their spins in opposite directions. The p orbital only has the 1 electron, by itself with no pair. This question is not asking about the number of valence electrons.
|
|
|
Suppose that CH4 (g) reacts completely with O2 (g) to form CO2 (g) and H2O (g) with a total pressure of 1.2 torr. What is the partial pressure of H2O(g)?
|
The answer is 0.8 torr.
The balanced equation for the complete combustion of CH4 (g) is: CH4 (g) + 2O2 (g) yields CO2 (g) + 2H2O (g) The pressure of the gaseous products is 1.2 torr. For every 3 product molecules, 2 are water. Therefore, the partial pressure of water is 2/3 the total pressure, because the total pressure is a function of the total number but not the kind of molecules (a colligative property). 2/3 of 1.2 torr is 0.8 torr. Thus, 0.8 torr is the answer. Alternate solution: The total pressure is Ptotal = PCO2 + PH20. Stoichiometrically, the number of moles of CO2 formed is 1/2 the number of moles of H20 formed. The partial pressure of each gas depends only on the number of moles. Then, PCO2 = (0.5)* PH20, and Ptotal = (0.5)* PH20 + PH20 = (1.5) * PH20 = 1.2 torr. Therefore, PH20 = 0.8 torr. |
|
|
Which of the following species has the largest radius?
A) Cl B) Cl- C) Al3+ D) Na+ E) F- |
The answer is Cl-. Because NA+ leads to the configuration of a noble gas above Cl-'s configuration of a noble gas, it has a smaller radius.
|
|
|
The first ionization energy of C is 11.3 eV. The first ionization energy of Si should be:
A) less than 11.3 eV. B) greater than 11.3 eV. C) 11.3 eV. |
A) less than 11.3 eV.
Because Si is a larger atom than C, it will take less energy to remove an electron than it will for C. |
|
|
will show an exceptionally large increase over the preceding ionization energy?
A) 2nd B) 3rd C) 4th D) 5th E) 6th |
The answer is A) 2nd
The reason is that Sulfer will have a half filled p orbital at the p^3 spot after the first ionization energy. It's stable there and is really not going to want to move to the p^2 spot with a second ionization energy. Don't forget about stable half filled p and d orbitals in regards to ionization energy and electron affinity! |
|
|
Deviations from the ideal gas law are smaller at:
A) low temperatures and high pressures. B) low temperatures and low pressures. C) high temperatures and high pressures. D) high temperatures and low pressures. |
Read the question closely. Deviations are SMALLER at what? Well, deviations are bigger at low temperatures and high pressures, but that's not the question. When are deviations smaller? The opposite! High temperature and low pressure!
|
|
|
If you have a piece of metal of 100g at 215 degrees celsius, it's exact melting point, and you hold it for a fraction of a second under a Bunsen burner, what will happen?
|
A small amount of the metal will melt, but the temperature will remain the same. Melting occurs at constant temperature because a certain amount of energy, the latent heat of fusion, is needed to convert the solid to its liquid state. The temperature of the melt will not increase above its melting point until all of the metal is melted. The small amount of heat could not melt 100g of the metal, but it could melt a small amount of the metal at the constant temperature of the melting point.
|

