![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
living things are made of.... |
Chemical compounds |
|
|
|
Atom |
Basic unit of matters |
|
|
|
Subatomic particles |
Protons, neutrons, electrons |
|
|
|
Nucleus |
Protons and neutrons; center of atom and cell |
|
|
|
Elements |
A pure substance that consists entirely of one type of atom; more than 100 elements are known |
|
|
|
Isotopes |
Atoms of the same element that differ in the number of neutrons they contain |
|
|
|
Why do all isotopes of an element have the same chemical properties? |
Because they have the same number of electrons |
|
|
|
Radioactive isotopes |
Isotopes' nuclei are unstable and break down at a constant rate over time; these isotopes give off radiation |
|
|
|
Compounds |
A substance formed by the chemical combination of two or more elements in definite proportions; written as chemical formulas |
|
|
|
Chemical bonds |
Ionic, covalent, and hydrogen bonds |
|
|
|
Ionic bonds |
Formed when one or more electrons are transferred from one atom to another |
|
|
|
Ions |
Positively and negatively charged atoms |
|
|
|
Covalent bond |
Forms when electrons are shared between atoms |
|
|
|
Molecule |
The smallest unit of most compounds; structure that results when molecules are joined together by covalent bonds |
Example: water |
|
|
van der Waals forces |
A slight attraction that develops between the oppositely charged regions of nearby molecules (happens in polar molecules such as water) |
|
|
|
Is ice denser than water? |
No; it's less dense, which is why it floats on water. |
|
|
|
Is a water molecule neutral? |
Yes; but its oxygen side is slightly negative while its hydrogen side is slightly positive. |
|
|
|
What does it mean when someone says water is a polar molecule? |
There is an unequal distribution of electrons between the oxygen and hydrogen atoms in a water molecule, which is why a water molecule is polar. |
|
|
|
Which side of water is negative and which is positive? |
The oxygen side is slightly negative, while the hydrogen side of a water molecule is slightly positive. |
|
|
|
What are hydrogen bonds? |
Weak bonds that are formed between water molecules; bonds between the negative side of one atom and the positive side of another |
|
|
|
Cohesion |
An attraction between molecules of the same substance (water bonding with water); water is extremely cohesive because of hydrogen bonding; cohesion is why water droplets collect into puddles or beads |
|
|
|
Adhesion |
An attraction between molecules of different substances; example: water sticking to glass |
|
|
|
Capillary action |
Adhesion between water and glass causes water to rise in a narrow tube against the force of gravity; one of the forces that draw water out of the roots of a plant and up into its stems and leaves while cohesion holds the column of water together as it rises |
|
|
|
Mixture |
A material composed of two or more elements or compounds that are physically mixed together but not chemically combined; ex: salt and pepper stirred together Two types of mixtures that can be made with water are solutions and suspensions |
|
|
|
Solution |
Mixture of two or more substances in which the molecules of the substances are evenly distributed |
|
|
|
Solute |
The substance that is dissolved Ex: table salt in a saltwater solution |
|
|
|
Solvent |
The substance in which the solute dissolves Ex: water in a saltwater solution |
|
|
|
Suspensions |
Mixtures of water and nondissolved materials |
|
|
|
What is the pH of water? |
Water has a pH of 7, so it is neutral |
|
|

pH scale |
.Ranges from 0 to 14 .at a pH of 7, the concentration of H+ ions and OH- ions is equal (pure water has a pH of 7) .solutions with a pH below 7 are acidic because they have more H+ ions than OH- ions .Solutions with a pH above 7 are basic because they have more OH- ions than H+ ions .Each step on the pH scale represents a factor of 10 |
|
|
|
Acid |
Any compound that forms H+ ions in a solution; acidic solutions contain higher concentrations of H+ ions than pure water and have pH values below 7 .strong acids tend to have pH values that range from 1 to 3 |
|
|
|
Base |
A compound that produces hydroxide ions (OH- ions in solution) .Basic (alkaline) solutions contain lower concentrations of H+ ions than pure water and have pH values above 7 .Strong bases such as lye tend to have pH values ranging from 11 to 14 |
|
|
|
Buffers |
Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in pH .controlling pH is important for maintaining homeostasis |
|
|
|
Chemistry of Carbon |
.carbon atoms have 4 valence electrons .can form strong covalent bonds .can bond to other carbon atoms, so carbon can form chains that are almost unlimited in length: can be single, double, or triple covalent bonds .versatile, can form millions of different large and complex structures |
|
|
|
Macromolecules |
4 macromolecules: proteins, nucleic acids, lipids, carbohydrates |
|
|
|
Monomers |
Smaller units of macromolecules |
|
|
|
Polymers |
Formed by joining monomers together |
|
|
|
Carbohydrates |
.Compounds made up of carbon, hydrogen, and oxygen atoms, usually in a ratio of 1:2:1 .used as a main source of energy, short term energy storage, structural support |
|
|
|
Monosaccharides |
The monomer units of carbohydrates Single sugars |
|
|
|
Polysaccharides |
Polymer units of carbohydrates Composed of monosaccharides Ex: statch |
|
|
|
Glycogen |
Provides energy |
|
|
|
Lipids |
.made mostly from carbon and hydrogen atoms .can be used for long term energy storage, important parts of biological membranes and waterproof coverings, hormones Ex: oils, steroids, fats .triglycerides: have a glycerol backbone connected to fatty acid chains |
|
|
|
Fatty acids |
Saturated fatty acids: no double bonds, usually solid at room temp Unsaturated fatty acids: double bond, usually liquid at room temperature (ex oil) |
|
|
|
Nucleic acids |
.macromolecules containing hydrogen, oxygen, nitrogen, carbon, and phosphorus .store and transmit hereditary or genetic information |
|
|
|
Nucleotides |
Monomer units of nucleic acids Contain a pentos sugar, nitrogenous base, and phosphate group |
|
|
|
Nitrogenous bases |
A denine T hymine C ytosine G uanine [uracil instead of thymine] |
|
|
|
Two kinds of nucleic acids |
DNA (deoxyribonucleic acid) RNA (ribonucleic acid) |
|
|
|
Proteins |
.Macromolecules that contain nitrogen as well as carbon, hydrogen, and oxygen .monomer units are amino acids, which have an amino group (NH2) on one end and a carboxyl group on the other end (COOH) . Over 20 dif. amino acids found in nature, identical in the regions where they may be joined together by covalent bonds .any amino acid can be joined to any other amino acid by bonding an amino group to a carboxyl group .each protein has a specific role . Some control the rate of reactions or speed them up and regulate cell processes; others are used to form bones and muscles; others transport substances into or out of cells or help to fight diseases |
|
|
|
Chemical Reactions |
Process that transforms one set of chemicals into another .mass and energy are conserved during chemical transformations |
|
|
|
Reactants |
The elements or compounds that enter into a chemical reaction |
|
|
|
Products |
Elements or compounds produced by a chemical reaction |
|
|
|
What do chemical reactions always involve? |
Changes in the chemical bonds that join atoms in compounds |
|
|
|
Energy in reactions |
Energy is released or absorbed whenever chemical bonds form or are broken |
|
|
|
Chemical Reactions and energy |
Chemical reactions that release energy often occur spontaneously Chemical reactions that absorb energy will not occur without a source of energy Released in form of heat |
|
|
|
Activation energy |
The amount of energy needed to get a reaction started |
|
|
|
Enzymes |
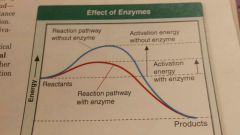
Proteins that act as biological catalysts .they speed up chemical reactions that take place in cells by lowering the activation energy |
|
|
|
Catalyst |
A substance that speeds up the rate of a chemical reaction, work by lowest a reaction's activation energy |
|
|
|
Substrates aka reactants |
The reactants of enzyme-catalyzed reactions |
|
|
|
Lock and key mechanism |
Certain enzymes can only react with certain types of substrates Metaphorically, the substrate is a key while the enzyme is a lock The active site, the place where the substrate attaches to the enzyme, is the groove in the lock |
|
|
|
High temperatures and pH affect enzymes how? |
Denature active site and make the enzyme unable to react with substrate Also affected by salinity |
|
|
|
Cold temperatures affect reactions by..? |
Slowing down enzyme movement and lessening reactivity with substrate |
|

