![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
define fluorescence?
|
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.
|
|
|
Is the emission or absorption wavelength longer?
|
emission
|
|
|
How long does a fluorescence molecule hang out in the excited state?
|
nanoseconds
|
|
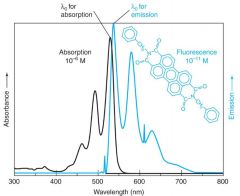
Fluorescence emission spectra are roughly the ____________ of their absorption spectra.
|
mirror image
|
|
|
Fluorescence emission is ______________ dependent.
|
solvent
|
|
|
Flourescence ___________ is solvent dependent.
|
emission
|
|
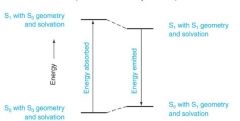
This is why the So transistions for the _________ spectrum and ___________ spectrum do not always overlap.
|
absorption, emission (fluorescence)
|
|
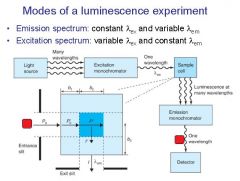
|
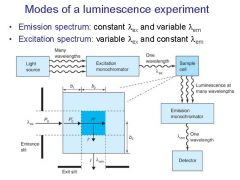
|
|
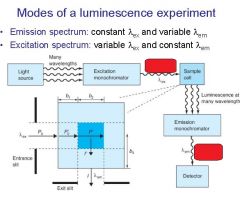
|
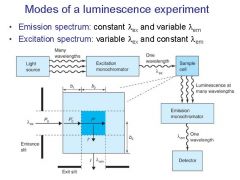
|
|
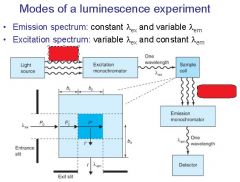
|
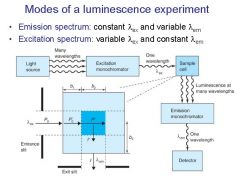
|
|
|
|
|
|
|
|
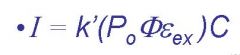
I=
|
fluorescent intensity
|
|
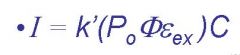
k'=
|
instrumental parameters, must be calculated
|
|
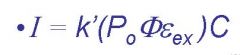
Po=
|
incident light, molecule dependent
|
|
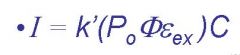
ɸ=
|
efficiency of flourescent quantum yield
|
|
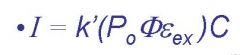
Eex=
|
molar absorptivity at Po
|
|
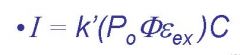
C=
|
concentration
|
|
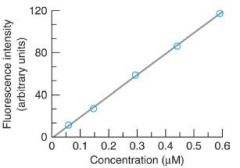
Slope =
|
k'(PoɸEex)
|

