![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
What are bacteriocins?
|
They are antimicrobial proteins that are produced by microbes
|
|
|
What are the two types of protein food preservatives?
|
Bacteriocins, antimicrobial proteins naturally present
|
|
|
What type of protein is lysozyme, what type of food is it found in, what are a few of its charactetrisitics, what is it's antimicrobial mechanism, how does it degrade cell wall?
|
Lysozyme is an antimicrobial protein naturally present in food
Found in eggs (3.5% of egg protein) Is a small protein that is heat stable and active mostle against gm+ protein lyses cell wall Protein can be used against gm- bacteria if an EDTA (chemical that chelates (binds) cations that are in LPS) treatment is applied lysozyme degrades cell wall by cleaving B-1,4 glycosidic bond between molecules (is an O molecule) |
|
|
What does Lactoferrin do, where is it found, what is the mechanism by which it works, when will it work?
|
Lactoferrin is an iron binding glycoprotein that requires bicarbonate to function.
Found in milk, tears and saliva. Binds two molecules of iron when bicarbonate is present, only will work when iron is limiting (Fe is limiting when pH is neutral since is insoluble in non-acidic conditions). |
|
|
How does avidin work? What molecule does it bind to? Where is it found?
|
It is bacteriostatic inhibitor that primarily prevents microbial growth by preventing them from getting biotin.
Also may inhibit bacteria by binding to transport proteins and interrupting transport Avidin is found in egg white protein |
|
|
Which of these are preservatives are resistant to heat? Transferrin, Lactoferrin, Lysozyme. Why?
|
Lysozyme. Because of its small size.
|
|
|
How is transferrin bacteriostatic to bacteria? What does it do and how does it work? Where is it found?
|
Transferrin is found in meats and binds iron, thus creating an environment low in free iron, where few bacteria are able to survive. It is most effective at a pH of neutral and effectiveness decreases as food is more acid.
|
|
|
What is ovotransferrin?
|
Is similar to transferrin except it is a major egg protein.
|
|
|
What are bacteriocins, why are they produced, what is their affected range, which bacteria have the widest range, what are the classes?
|
Bacteriocins are small protein compounds produced by some bacteria that are inhibitory to others
They are produced to ward off competitors. Most only affect the same genus, just different species. Lactic Acid bacteria have bacteriocins that affect the widest range of bacteria Two classes are lantibiotics (I) and (II) non-post translationally modified. |
|
|
What are the chemical characterisitcs of the lantibiotics and what are the most important ones?
|
Lantibiotics are characterized by unusual amino acids with lanthionine rings that are formed after translation
Nisin and Lacticin 3147 are two examples |
|
|
Where does Nisin come from, where and how is it used?
|
Comes from Lactococcus lactis, is bacteriocidal, kills most gm + bacteria, used as a starter culture in cheese, has broadest inhibitory ability of the lantibiotics.
|
|
|
What is the mechanism of Nisin in killing bacteria?
|
Is eaten and digested
Forms pores in cell membrane in hexagon shapes Binds and uses Lipid II to facilitate anchoring the membrane Depletes PMF, which causes the cell to lose charge and die |
|
|
Since Nisin is not a GRAS substance, how are companies getting around the legislation to use it as a preservative
|
Lactobacillus lactis is GRAS. So the bacteria is added to whey, as an example, and produces Nisin. The whey is then dried. Companies can sell the whey as a proprietary blend advertising it as a shelf life extender and not disclosing the ingredients.
|
|
|
How is Nisin production regulated?
|
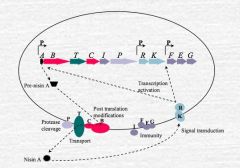
B&C are post translation modification genes
T is for transport P is for protease cleavage R&K are for regulation (R activates K when there is an appropriate Nisin level in the environment) I, F, E, G are immunity genes that protect the cell Basically, the pre-nisin has an extra peptide attached to the N terminal. This is cleaved by the protease gene and released from the cell. The finished nisin then activates the K gene which in turn activates the R gene via phosphorilation and the cycle repeats. |
|
|
When is nicin effective? When is lacticin 3147 effective?
|
acidic pH, neutral pH
|
|
|
What are characteristics of bacteriocins that are not post translationally modified?
|
characterized by a glycine-glycine leader sequence that helps protect protein from transport through the cell membrane. once inside, leader sequence is shut off
they are heat stable, small |
|
|
What bacteria produces PEDIOCIN, what bacteria is it effective against, what is mode of action that kills bacteria?
|
Pediococcus produces PEDIOCIN
Very effective in control of Listeria Mode of action is pore formation, and depletion of Proton motive force, not well characterized |

