![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
196 Cards in this Set
- Front
- Back
|
Body fluid compartments
a. Total fluid % b. The 5 different fluid compartments and their relative proportions |
a. 60%
b. 1. ICF: 2\3 2. ECF: 1\3 a. Interstitial (ISF): 3\4 of ECF b. Plasma volume (PV): 1\4 of ECF (Netter flash cards) |
|
|
Daily production of water from oxidative metabolism of carbohydrates
a. Amount |
a. 200 mL\day
(Guyton) |
|
|
Insensible water loss
a. What b. Amount c. Do these increase with exercise? |
a. Loss of water that we are not consciously aware of, via evaporation from the respiratory tract and diffusion through the skin. Occurs independently of sweating (present in people born without sweat glands)
b. 700 mL\day: 350 mL\day via skin and 350 mL\day via lungs c. Only the insensible loss via lungs (The part via skin can be increased to 5L\day with extensive burns) (Insensible loss via lungs is due to the fact that the vapor pressure of expired air, which is 47 mmHg, is higher than that of inspired air, which can be 0 mmHg at cold days) (Guyton) |
|
|
Water loss due to sweat
a. Normal b. Up to during heavy exercise or hot weather |
a. 100 mL\day
b. 2L\hour (Guyton) |
|
|
Water loss in feces
a. Normal b. Up to during severe diarrhea |
a. 100 mL\day
b. Several liters a day (Guyton) |
|
|
Water loss by kidneys
a. Minimum amount in dehydrated person b. Maximum amount |
a. 0.5L\day
b. 20L\day (Guyton) |
|
|
Transcellular fluid
a. What b. Sometimes considered part of |
a. Synovial, peritoneal, pericardial, pleural, intraocular, cerebrospinal..
b. 1-2L (Guyton) |
|
|
Average blood volume
a. % of body weight b. Amount |
a. 7% of body weight
b. 5L (Guyton) |
|
|
Hematocrit
a. What b. Why is the true hematocrit only 96% of the measured hematocrit c. Value in men and vomen d. Lowest compatible with life |
a. The fraction of the blood composed of red blood cells (Packed red cell volume)(determined by centrifugating blood in a "hematocrit tube")
b. Since it is impossible to completely pack the red cells together, about 3-4% of the plasma remains entrapped among the cells c. Men: 0.4, Women: 0.36 d. 0.10 (during severe anemia) (Can be as high as 0.65 with polycythemia) (Guyton) |
|
|
Gibbs-Donnan effect
a. What b. Example |
a. Name for the behavior of charged particles near a semi-permeable membrane to fail to distribute evenly across the two sides if there is some non-permeable substance that is charged
b. Account for the 2% increase in cations in the plasma compared to the interstitial fluid. Due to non-permeable protein anions in the plasma. |
|
|
Which 4 substances has a higher intracellular than extracellular concentration?
|
1. K+
2. Protein (almost 4 times as much) 3. Phosphate 4. Mg2+ (Guyton) |
|
|
Indicator dilution method
a. Formula b. Requirements |
a. Volume (mL) = injected indicator (mg)\concentration (mg\mL)
b. The indicator 1. Disperses evenly 2. Does not get metabolized\excreted (Guyton) |
|
|
Body fluid compartments - dyes for indicator dilution method
a. For total body water b. For extracellular fluid c. For intracellular fluid d. for plasma volume e. Interstitial fluid f. Blood volume |
a. Radioactive water\tritium, heavy water\deuterium, antipyrine (very lipid soluble)
b. Inulin, radioactive sodium (22-Na), Radioactive chloride c. Don't use dye for it. Intracellular volume = Total body water - extracellular volume d. Albumin labeled with radioactive iodine (125-I- albumin), Evans blue dye (bind avidly to plasma proteins)) e. Interstitial volume cannot be measured directly. Interstitial volume = extracellular fluid volume - plasma volume f. Total blood volume = plasma volume\1-hematocrit, inject rbc marked with radioactive Chromium (Guyton) |
|
|
Osmosis
|
The net diffusion of water down its concentration gradient across a selectively permeable membrane
(Guyton) |
|
|
One osmole equals?
|
One mole (6.02 x 10^23) of solute\osmotically active particles
(Guyton) |
|
|
Osmotic pressure
a. What b. Relation to osmolarity c. Van't Hoff's law d. With normal body temperature, a solution with 1 mOsm\L has osmotic pressure of |
a. The amount of pressure required to prevent osmosis
b. Directly proportional c. π = C R T, π = Osmotic pressure (in mmHg) C = Osmolarity R = Ideal gas constant T = Absolute temperature (Kelvin (273 + centigrade)) d. With normal body temperature, a solution with 1 mOsm\L has osmotic pressure of 19.3 mmHg (Osmotic coefficient: constant for solutes which take into account the effect of their interionic and intermolecular interactions. 0.93 for sodium chloride) (Guyton) |
|
|
Osmolarity
a. Of plasma b. Of interstitial fluid (and ICF) c. Why, implications |
a. 282 mOsm\L
b. 281 mOsm\L c. Due to plasma proteins. Since 1 mOsm\L = about 20 mmHg in osmotic pressure, the capillaries maintain about a 20 mmHg higher pressure than the interstitial fluid. (Guyton) |
|
|
Examples of isotonic solutions (2)
|
1. 0.9% solution of sodium chloride
2. 5% glucose solution (These are important in medicine because they can be infused without the danger of upsetting osmotic equilibrium between the intracellular and extracellular fluids) (Guyton) |
|
|
Difference between (hypo-, iso-, hyper- tonicity and osmotic (hypo-, iso-, hyper-) fluids
|
The tonicity of a solution depends on the concentration of impermeable solutes.
-Osmotic solutions refer to the level of solutes, regardless of whether they can penetrate the cell membrane. (Guyton) |
|
|
Sodium and its associated anions in the ECF account for more than ... % of the solute in the ECF
|
90
(Therefore plasma sodium concentrations is a reasonable indicator of plasma osmolarity under many conditions) (Guyton) |
|
|
Intracellular edema
a. 3 causes b. Mechanism |
a.
1. Depression of the metabolic systems 2. Lack of adequate nutrition (hypoxia, tromboembolic states) 3. Inflammation b. 1&2: ↓energy -> ↓Na-K-ATPase -> ↑Na+ intracellularly -> cause osmosis 3: Inflammation have a direct effect on the cell membranes to increase their permeability, thus allowing Na+ and other ions to diffuse to the interior of the cell and cause osmosis of water into the cell (Guyton) |
|
|
Starling equation
a. Starling forces b. Starling equation |
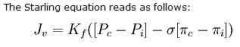
a. Oncotic & hydrostatic pressures
b. Image Jv is the net fluid movement between compartments. Capillary hydrostatic pressure ( Pc ) Interstitial hydrostatic pressure ( Pi ) Capillary oncotic pressure ( πc ) Interstitial oncotic pressure ( πi ) Filtration coefficient ( Kf ) = capillary surface area x capillary hydraulic conductance Reflection coefficient ( σ ) Correction factor - account for the decreased oncotic pressure of the plasma due to a slight leakage of plasma proteins causing decreased plasma oncotic pressure & increased interstitial fluid oncotic pressure (Account for the fact that the effective capillary oncotic pressure is lower than the measured capillary oncotic pressure) (Glomerular capillaries have a reflection coefficient close to 1 as normally no protein crosses into the glomerular filtrate, while the hepati sinusoids have a low reflection coefficient as they are quite permeable to protein. Useful since they make plasma proteins..) (cvphysiology.com) |
|
|
Causes of extracelular edema
a. Increased capillary pressure (3) b. Decreased plasma proteins (3) c. Increased capillary permability (6) d. Blockage of lymph return (4) |
a. Increased capillary pressure
1. Excessive kidney retention of salt and water (acute\chronic kidney failure, Cushing's syndrome, Conn disease) 2. High venous pressure and venous constriction (heart failure, venous obstruction, failure of venous pumps (valves, muscle paralysis, immobilization)) 3. Decreased arteriolar resistance (hyperthermia, insufficiency of sympathetic nervous system, vasodilators) b. Decreased plasma proteins (severe when plasma proteins is < 25g\L) 1. Nephrotic syndrome 2. Denuded skin areas (burns, wounds) 3. Failure to produce proteins (cirhossis, protein deficiency, caloric deficiency) c. Increased capillary permeability 1. Immune reactions 2. Toxins 3. Bacterial infections 4. Hypovitaminosis - esp. vitamin C 5. Prolonged ischemia 6. Burns d. Blockage of lymph return 1. Cancer 2. Infections - filaria nematoda (-> elephant leg\elephantiasis) 3. Surgery (radical mastectomy) 4. Congenital absence\abnormality of lymphatic vessels (Edema due to lymphatic blockage can become very severe. Leaked plasma proteins have no place to be removed -> progressive edema) (Guyton) |
|
|
Compliance
|
The change in volume per mmHg change.
(Guyton) |
|
|
Safety factors that normally prevent edema
a. Which(3), numerical values b. Total safety factor, value |
a.
1. Low compliance of the interstitium in the negative pressure range -> 3 mmHg 2. The ability of lymph flow to increase 10- to 50-fold -> 7 mmHg 3. Washdown of interstitial fluid protein concentration -> 7 mmHg b. 17 mmHg (Thus the capillary pressure in a peripheral tissue could theoretically rise by 17 mmHg \ almost double the normal value before marked edema would occur) (Guyton) |
|
|
Edema - safety factor caused by low compliance of the interstitium in the negative pressure range
a. What is the interstitial fluid hydrostatic pressure in most loose subcutaneous tissue? b. How big is the safety factor against edema? c. Why is there a difference in the complience above and below 0 mmHg |
a. -3 mmHg (This slight suction in the tissues helps hold the tissues together)
b. 3 mmHg. The interstitial fluid must increase 3 mmHg up to 0 mmHg before large amounts of fluid will begin to accumulate in the tissues (Guyton) |
|
|
Edema - safety factor caused by low compliance of the interstitium in the negative pressure range
a. Why is there a difference in the complience above and below 0 mmHg |
a. Below zero the tissue architecture is maintained in gel form by proteoglycan meshworks. This
1. Prevent fluid from flowing easily through the tissues (because of impediment from the "brush pile" of proteoglycan filaments) 2. During very negative interstitial fluid pressure it does not contract greatly because of its elastic nature -> thus the interstitial fluid volume does not change greatly Normally, virtually all the fluid in the interstitium is in gel form -- bound in a proteoglycan meshwork so that there are virtually no significant free fluid spaces. Above 0 mmHg there is a big accumulation of free fluid in the tissues. This happens because the extra fluid pushes the brush pile of proteoglycans apart (Guyton) |
|
|
Edema
a. Pitting edema: <-, why pitting b. Nonpitting edema, <- |
a. Pitting edema
Occurs when free fluid accumulates when the interstitial fluid pressure is above 0 mmHg. Pitting because one can press the thumb against the tissue area and push the fluid out of the area, when the thumb is removed, a pit is left in the skin for a few seconds until the fluid flows back from the surrounding tissues b. Nonpitting edema 1. When the tissue cells swell 2. When the fluid in the interstitium becomes clotted with fibrinogen so that it cannot move freely within the tissue (Guyton) |
|
|
Kidneys - functions (7)
|
1. Excretion of metabolic waste products and foreign chemicals (urea <- AAs, creatinine, uric acid, end products of hemoglobin breakdown, metabolites of various hormones)(pesticides, drugs, food additives)
2. Regulation of water 3. Regulation of electrolytes and osmolarity 4. Regulation of arterial pressure 5. Regulation of acid-base balance 6. Secretion, metabolism, and excretion of hormones (EPO, renal 25-hydroxycholecalciferol 1-alpha-hydroxylase) 7. Gluconeogenesis (Guyton) |
|
|
The renal circulation is unique because
|
It has two capillary beds, the glomerular and peritubular capillaries, which are arranged in series and separated by the efferent arterioles.
(Guyton) |
|
|
Nephrons
a. Number of nephrons in each kidney b. Types c. After age 40, the number of functioning nephrons usually decrease about ... % every 10 years |
a. 800 000-1 million
(The kidney cannot regenerate new nephrons) b. Juxtamedullary (have vasa recta) (25-30%) and cortical (all in cortex) c. 10 (Guyton) |
|
|
Hydrostatic pressure of glomerular capillaries
|
60 mmHg
(Guyton) |
|
|
Draw a juxtamedullary nephron - include all tubular structures, afferent & efferent capillaries & glomerulus
|
|
|
|
Draw a juxtamedullary nephron - include all tubular structures, afferent & efferent capillaries & glomerulus
|
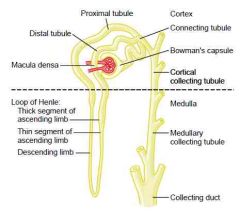
|
|
|
Macula densa
a. Location |
a. In the end of the thick ascending limb of loop of Henle (The portion after is called the distal convoluted tubule)
|
|
|
Vasa recta
a. Associated with which nephrons b. What |
a. Juxtamedullary nephrons
b. straight vessels into which the efferent arteriole branches up; they form a bundle of vessels which, arising at the bases of the pyramids, run through the renal medulla toward the apex of each pyramid, then reverse direction in a hairpin turn, and run straight back again toward the base of the pyramid as venae rectae |
|
|
Glomerulus
a. Synonym b. What |
a. Malphigian glomerulus\Renal corpuscle
b. The tuft (cluster) of glomerular capillaries and the glomerular capsule\Bowman capsule that surrounds it (Guyton) |
|
|
Nephron - components
|
Renal corpuscle -> DCT
|
|
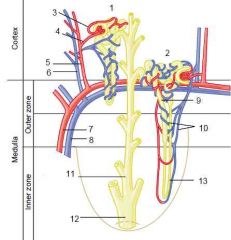
|
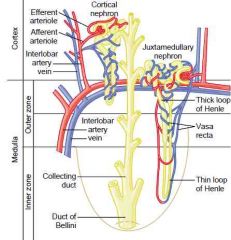
Bellini ducts\Papillary ducts: the largest excretory ducts in the kidney medulla and papillae whose openings form the area cribrosa that open into a minor calyx, they are continuation of collecting tubules
|
|
|
Detrusor muscle
|
The smooth muscle of the bladder.
(Guyton) |
|
|
Sphincters of the bladder
a. Internal sphincter - where, smooth\skeletal, innervated by |
a. Internal sphincter
In bladder neck, smooth, parasympathetics (S2-3) b. External sphincter In urogenital diaphragm, skeletal, pudendal nerve (Guyton) |
|
|
Ureters
a. How is urine prevented from going from the bladder to the ureters b. What is the name of the condition where the distance that the ureter courses through the bladder wall is reduced, so that during micturition some of the urine in the bladder is propelled backward into the ureter? |
a. The ureters course obliquely for several centimeters through the bladder wall. The normal tone of the detrusor muscle compress the ureter.
b. Vesicoureteral reflux (can lead to enlargement of ureters and in severe cases increase pressure in the renal calyces and structures of the renal medulla, causing damage to these regions) (Guyton) |
|
|
Ureterorenal reflex
|
Ureter obstruction -> pain impulses -> sympathetic reflex back to the kidney to constrict the renal arterioles -> ↓urine output
(Guyton) |
|
|
At what volume level of urine does the intravesicular pressure begin to rise steeply?
|
300-400 mL
(Guyton) |
|
|
Micturition reflex
a. What b. How is it related to increase in intravesical pressure c. Sensory component d. Components of the reflex arch |
a. Cycle of
1. Progressive and rapid increase of pressure 2. A period of sustained pressure 3. Return of the pressure to the basal tone b. It increase in frequency and strength as the intravesical pressure increase c. Stretch receptors in the bladder wall, especially in the posterior urethra\neck d. Sensory -> pelvic nerves -> sacral segments -> parasympathetics via pelvic nerves (After an unsuccessful event the nervous elements is in an inhibited state for a few minutes to an hour) (Once it becomes powerful enough it causes another reflex which passes through the pudendal nerves to the external sphincter to inhibit it) (Guyton) |
|
|
Facilitation or inhibition of micturition by the brain
a. Centers |
a.
1. Facilitative and inhibitory centers in the brain stem, mainly in the pons 2. Several centers in the cerebral cortex, mainly inhibitory b. Effects 1. Keep the micturition reflex partially inhibited 2. Prevent micturition by external sphincter 3. The cortical center can facilitate the sacral micturition centers to help initiate a micturition reflex (Guyton) |
|
|
Urinary excretion rate formula
|
Urinary excretion rate = filtration rate - reabsorption rate + secretion rate
(Guyton) |
|
|
GFR
a. \day b. \min |
a. 180L\day
b. 125mL\min (Guyton) |
|
|
Which substances do not appear in the glomerular filtrate in the same concentrations as in the plasma?
|
1. Most proteins
2. Cellular elements 3. Ca2+ (50%), Fatty acids (most) -> bound to plasma proteins (Guyton) |
|
|
Glomerular filtration barrier
a. Components (3) b. Special characteristics (3) |
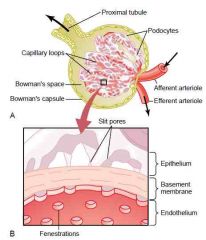
a.
1. Endothelium 2. Basement membrane 3. Podocytes (epithelium) b. Special characteristics 1. Endothelium has fenestrae (holes) 2. Negative charges preventing filtration of plasma proteins in endothelium & podocytes (richly endowed with fixed negative charges) and by proteoglycans in the basement membrane 3. Slit pores between foot processes of podocytes (Guyton) |
|
|
Filterability
a. What b. kD and filterability of myoglobin c. kD and filterability of albumin d. filterability of inulin |
a. A filterability of 1.0 means that the substance is filtered as freely as water
b. 17 kD, 0.75 filterability c. 69 kD, 0.005 filterability d. 1.0 filterability (5.5 kD) (Guyton) |
|
|
What is the name of the condition where the negative charges on the basement membrane are lost thus allowing protein filtration, followed by progression to other kidney diseases
|
Minimal change nephropathy
(Guyton) |
|
|
GFR
a. Net filtration pressure b. Glomerular hydrostatic pressure c. Bowman's capsule pressure d. Glomerular oncotic pressure e. Bowman's oncotic pressure |
a. 10 mmHg
b. 60 mmHg c. 18 mmHg d. 32 mmHg (normally 28 mmHg, increase to 36 mmHg in the efferent arteriole. 32 mmHg is an average) e. 0 mmHg |
|
|
Filtration coefficient
a. Components (2) b. Formula (derived from other) c. Value in renal corpuscle d. Function in regulation of GFR |
a. Hydraulic conductivity & surface area of glomerular capillaries
b. GFR = Kf x Net filtration pressure -> Kf = GFR\NFP c. 12.5 mL\min\mmHg (NFP is 10 mmHg, GFR is 125 mL\min)(4.2mL\min\mmHg\100g kidney. 400 x most other capillaries which are 0.01mL\min\mmHg\100g) d. Probably not a primary mechanism for day-to-day regulation of GFR (Decreased in chronic uncontrolled hypertension and DM by increasing thickness of glomerular capillary basement membrane and thus decreasing hydraulic conductivity and eventually by damaging the capillaries so there is loss of capillary function) (Guyton) |
|
|
Filtration fraction
a. Formula b. How can ↓renal blood flow decrease GFR without decreasing glomerular hydrostatic pressure? |
a. Filtration fraction = GFR\Renal blood flow
b. ↓Renal blood flow -> ↑FF -> ↑Glomerular oncotic pressure -> ↓GFR (Guyton) |
|
|
Glomerular capillary hydrostatic pressure - 3 components, what is their effect
|
1. Arterial pressure -> ↑-> ↑GFR, but this is buffered by autoregulatory mechanisms (within MAP of 75-160 mmHg)
2. Afferent arteriolar resistance: ↑-> ↓GFR, ↓->↑GFR 3. Efferent arteriolar resistance -> biphasic a. Up to 1.5 increase -> ↑GFR b. > 1.5 increase -> ↓GFR due to ↑glomerular oncotic pressure <- damming of blood & ↓renal blood flow (caused by ↑resistance) (Guyton) |
|
|
Determinants of renal blood flow (Q = Pressure difference\resistance)
a. Most of the renal vascular resistance resides in three segments b. What is the general effect of ↑ or ↓ resistance in these? |
a. Interlobular artery (16%), afferent arteriole (26%), and efferent arteriole (43%)
b. ↑resistance -> compensatory ↓renal blood flow, while ↓resistance -> ↑renal blood flow (Guyton) |
|
|
Blood flow to renal medulla
a. Supplied by which vessels b. Percentage of renal blood flow |
a. Vasa recta
b. 1-2% (Guyton) |
|
|
Autocoid
|
The agents of paracrine secretions
|
|
|
Endothelin
a. What b. Secreted by c. Associated with which disease states (3) |
a. Autocoid vasoconstrictor peptide that may contribute to hemostasis (minimizing blood loss) when a blood vessel is severed
b. Damaged vascular endothelial cells c. Certain disease states associated with vascular injury 1. Toxemia of pregnancy 2. Acute renal failure 3. Chronic uremia (Guyton) |
|
|
Angiotensin II
a. Renal effect b. What is the consequence of this |
a. Preferentially constrict efferent arterioles -> ↑ -> ↑GFR while reducing renal blood flow
b. Secretion of ATII usually occurs in circumstances associated with hypotension\hypovolemia which tend to decrease GFR - ATII thus help to buffer this. Also: ↓renal blood flow (caused by ATII) -> ↑filtration fraction -> peritubular oncotic pressure -> tubular reabsorption (Thus maintain essential elements while excreting toxic metabolites during hypotension\hypovolemia) (Guyton) |
|
|
Four endogenous substances that increase GFR
|
1. Endothelial-derived nitric oxide
2. Bradykinin 3. PGE2 4. PGI2 (The last 3 probably has some effect in counteracting ↓GFR during strong sympathetic stimulation, but otherwise not important regulators. Under stressful situations such as hypovolemia\surgery, administration of NSAID can significantly reduce GFR) (Impaired NO production can be the cause of some patients hypertension partly by decreasing GFR) (Guyton) |
|
|
Juxtaglomerular complex - components
|
1. Macula densa cells in the initial portion of the distal tubule, specialized epithelial cells (have extensive GA suggesting secretory activity)
2. Juxtaglomerular cells in the afferent and efferent arteriole (Guyton) |
|
|
Tubuloglomerular feedback mechanism - ie if arterial pressure is decreased
|
↓Arterial pressure -> ↓glomerular hydrostatic pressure -> ↓GFR -> ↑PCT NaCl reabsorption -> ↓Macula densa [NaCl] or ↑flow rate->
↓Afferent arteriolar resistance, ↑Efferent arteriolar resistance -> ↑Glomerular hydrostatic pressure (Also intrarenal baroreceptors in the afferent arteriole that work on juxtaglomerular cells) (Work within 75-160 mmHg of MAP) (Guyton) |
|
|
Myogenic autoregulation - ↑arterial pressure cause vasoconstriction by?
|
↑Arterial pressure -> ↑wall stretch -> stretch of the vascular wall allows increased movement of Ca2+ into the cell -> vasoconstriction
(Guyton) |
|
|
What is the purpose of the tubuloglomerular feedback mechanism?
|
To ensure normal rate of sodium and water excretion is maintained, by ensuring a constant delivery of NaCl to the distal tubule
(Thus its not to regulate renal blood flow or GFR. 1. Increased amino acids (high-protein meal) or glucose (DM) -> increased proximal tubular reabsorption -> ↓NaCl in macula densa -> ↑GFR, despite of no initial change in GFR 2. Damage of PCT (<-mercury, tetracycline) -> ↓tubular reabsorption -> ↑NaCl in macula densa -> ↓GFR) (Guyton) |
|
|
Filtration - formula
|
Filtration = GFR x Plasma concentration
(Guyton) |
|
|
Transport of water and solutes from he interstitial fluid into the peritubular capillaries occurs by?
|
Ultrafilitration, a passive process mediated by hydrostatic and colloid osmotic forces (like the venous ends of most other capillaries)
(Guyton) |
|
|
Glucose transport in kidneys
a. Transport maximum for glucose \min b. Threshold - what, value |
a. 375 mg\min
(The filtered load of glucose is only about 125 mg\min (GFR x plasma glucose = 125 mL\min x 1 mg\mL) (Can be saturated by large increase of GFR or plasma glucose concentration) b. The filtered load of glucose at which glucose first begins to be excreted in the urine, 11.1M plasma glucose concentration if GFR remains constant at 125 mL\min (Guyton) |
|
|
Gradient-time transport
|
Transport of substances that are passively reabsorbed do not demonstrate a transport maximum, their rate of transport depends on the electrochemical gradient and the time that the substance is in the tubule (which in turn depends on the tubular flow rate)
(Guyton) |
|
|
Some actively transported substances also have characteristics of gradient-time transport
a. Example b. How |
a. Sodium reabsorption in PCT
b. The capacity of the basolateral Na+-K+ pump exceeds the rate of Na+ reabsorption. This happens because a significant amount of sodium transported out of the cell leaks back into the tubular lumen through the epithelial tight junctions . The rate of this depends on the permeability of the tight junctions and the interstitial physical forces (Guyton) |
|
|
Solvent drag
a. What b. How does this relate to the fact that organic solutes and ions is coupled to reabsorption of sodium |
a. Water's capability to carry with it some of the solutes as it moves by osmosis
b. Reabsorption of sodium -> water follows by osmosis -> Solvent drag of other solutes (Guyton) |
|
|
Passive diffusion of water in the renal tubules
a. Paths of reabsorption b. Degree of permeability in PCT & descending limb of loop of Henle, Ascending limb, Last parts (DCT, collecting tubules, collecting ducts) |
a. Transcellular or paracellular via tight junctions
b. 1. PCT & descending limb of loop of Henle: high 2. Ascending limb of loop of Henle: tight junctions is almost impermeable 3. Last parts (DCT, collecting tubules, collecting ducts): can be high or low depending on the presence of ADH (Guyton) |
|
|
Mechanisms by which chloride and urea reabsorption are coupled with sodium reabsorption
|
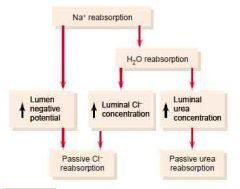
(Chloride is passively absorbed paracellularly, also have secondary-active transport with sodium across the luminal membrane)
(Urea is less permeable than chloride, so less is reabsorbed. 50% is excreted. There is reabsorption by facilitated diffusion in the inner medullary collecting duct by urea transporters) (Guyton) |
|
|
Proximal tubular reabsorption in %
Sodium, water, chloride, bicarbonate, , potassium, glucose, amino acids |
About 65% for sodium, water, chloride, bicarbonate, and potassium
(Can be regulated under different physiologic conditions) Essentially all the filtered glucose and amino acids are reabsorbed (Guyton) |
|
|
Loop of Henle
a. % Reabsorption of water, where b. % Reabsorption of sodium, chloride and potassium, where |
a. 20%, thin descending limb
b. 25%, mostly in thick ascending limb (Considerable amounts of other ions, such as calcium, bicarbonate, and magnesium are also reabsorbed here) (The thin descending limb is also moderatly permeable to most solutes but has few mitochondria and little or no active reabsorption) (Guyton) |
|
|
Thick ascending limb of loop of Henle
a. 3 important transport mechanisms b. All ultimately driven by |
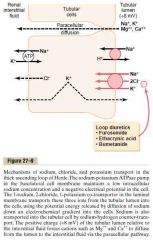
a.
1.Sodium 2-chloride potassium co-transporter 2. Sodium-hydrogen counter-transporter 3. Paracellular diffusion of Na, K, Mg, Ca.. b. Basolateral Na-K-ATPase (cause negative intracellular potential and low intracellular Na concentration) (The +8mV potential of the lumen is caused by slight back-leakage of K) (Its also virtually impermeable to water) (Guyton) |
|
|
Early distal tubule
a. Important transporter mechanisms b. % reabsorption of filtered load of NaCl c. The 2 parts of the early distal tubule |
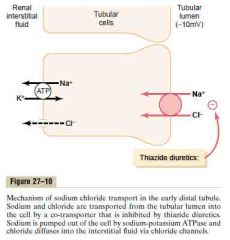
a. Sodium-chloride cotransporter
b. 5% c. First portion forms part of the juxtaglomerular complex, the next part is very convoluted and is similar to thick ascending limb of loop of Henle. (avidly reabsorbs most ions (Na, K, Cl, Ca, Mg) but is virtually impermeable to water and urea) (Guyton) |
|
|
Principal cells of late distal tubule and cortical collecting tubule
a. Functions (3) |
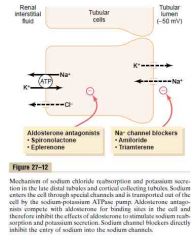
1. Have Na+ channel
2. Have extensive Na-K-ATPase -> ↑ IC K+ -> diffuse out -> lumen, renal interstitial fluid 3. Aldosterone has a stimulatory effect on sodium reabsorption and potassium secretion (Principal site of the potassium-sparing diuretics: spironolactone, eplerenone, amiloride, triamterene) (Sodium channel blockers -> block sodium influx -> decreased sodium to pump out by Na-K-ATPase -> less K is pumped inside) (90% of the cells in this region) (Guyton) |
|
|
Intercalated cells of late distal tubule and cortical collecting tubule - functions\characteristics (3)
|
1. H-ATPase
(Mediated by intracellular carbonic anhydrase: H2O + CO2 -> H2CO3 -> H+ + HCO3-. H+ is secreted while HCO3- is reabsorbed) 2. Can reabsorb K+ > (incompletely understood mechanism, has a luminal H-K ATPase that pump K in and H out, after K can diffuse out the basolateral membrane) 3. ADH can work on it (V2 receptors (G protein-coupled receptors on the basolateral membrane). Gs -> ↑adenylyl cyclases III and VI. The rise in cAMP then triggers the insertion of aquaporin-2 water channels by exocytosis of intracellular vesicles) (10% of the cells in this region) (Guyton & wikipedia) |
|
|
Medullary collecting duct - functions\characteristics
|
1. Permeable to urea (some of the urea is reabsorbed which helps to raise the osmolarity of the medullary interstitium, which further contributes to the kidneys' overall ability to concentrate urine)
2 Functions shared with intercaled cells of late distal tubules & cortical collecting tubules 1. H-ATPase (Mediated by intracellular carbonic anhydrase: H2O + CO2 -> H2CO3 -> H+ + HCO3-. H+ is secreted while HCO3- is reabsorbed) 2. ADH can work on it (V2 receptors (G protein-coupled receptors on the basolateral membrane). Gs -> ↑adenylyl cyclases III and VI. The rise in cAMP then triggers the insertion of aquaporin-2 water channels by exocytosis of intracellular vesicles) (Few mitochondria, only handles the last 10% of filtrate) (Guyton) |
|
|
Tubular fluid\Plasma ...... concentration ratio can be used to measure water reabsorption by the renal tubule
|
Inulin
(End of proximal tubule: 3 in ratio, end of collecting tubule: 125 in ratio) (Guyton) |
|
|
Glomerulotubular balance
a. Principle b. Where c. Function |
a. The ability of the tubules to increase reabsorption rate in response to increased tubular load
b. Mostly in proximal tubule, partly in loop of Henle c. Act as a second line of defense (after tubuloglomerular feedback) to buffer the effects of spontaneous changes in GFR on urine output (Guyton) (The proximal tubule can reabsorb 65% even though the GFR increase to 150 mL\min) |
|
|
What is the normal net reabsorption pressure of the peritubular capillaries?
|
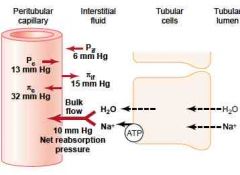
10 mmHg
(The filtration coefficient is also large - 12.4 mL\min\mmHg. As determined by Kf = reabsorption rate\reabsorption pressure) (Guyton) |
|
|
Hormonal control of tubular reabsorption - Aldosterone
a. Site of action b. Effects (2) |
a. Principal cells of late distal tubule and collecting tubule & duct
b. 1. Na reabsorption (with it water and Cl) b. K excretion (Guyton) |
|
|
Hormonal control of tubular reabsorption - Angiotensin II
a. Site of action b. Effects (2) |
a. All except thin parts of loop of Henle (thin descending, thin ascending) -> PT, thick ascending loop of Henle, DT, CT
b. 1. Na reabsorption (with it Cl and water) 2. H secretion (Guyton) |
|
|
Hormonal control of tubular reabsorption - ANP
a. Site of action b. Effect |
a. Late distal tubule, collecting tubule & duct
b. Decreased Na reabsorption (and with it Cl) by inhibitng eNAC (epithelial Na channel) |
|
|
eNAC (Epithelial sodium channel)
a. Synonym b. Renal location c. Stimulated by d. Inhibited by |
a. Amiloride-sensitive sodium channel (ASSC)
b. Primary in principal cells of late distal tubule and collecting tubule c. Aldosterone d. Atrial natriuretic factor, Sodium channel blockers: amiloride, triamterene (Guyton) |
|
|
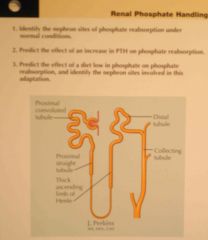
Hormonal control of tubular reabsorption - PTH
a. Site of action b. Effects (2) |
a. PT, thick ascending loop of Henle and early distal tubule
b. 1. ↓PO4 reabsorption 2. ↑Ca reabsorption (Guyton) |
|
|
Hormonal control of tubular reabsorption - Aldosterone
a. Site of action b. Effects (2) |
a. Principal cells of late distal tubule and collecting tubule & duct
b. 1. Na reabsorption (with it water and Cl) b. K excretion (Effects mediated by stimulating basolateral Na-K-ATPase and stimulate\increase eNAC) (Guyton) |
|
|
Hormonal control of tubular reabsorption - Angiotensin II
a. Site of action b. Effects (2) |
a. All except thin parts of loop of Henle (thin descending, thin ascending) -> PT, thick ascending loop of Henle, DT, CT
b. 1. Constrict efferent arterioles -> ↓peritubular capillary hydrostatic pressure, ↑filtration fraction (by ↑glomerular hydrostatic pressure) -> ↑peritubular capilllary oncotic pressure -> ↑reabsorptive force (Na, Cl, H2O) 2. Stimulate basolateral Na-K-ATPase 3. Stimulate Na-H exchange, especially in PT (Guyton) |
|
|
Hormonal control of tubular reabsorption - ANP
a. Site of action b. Effect |
a. Late distal tubule, collecting tubule & duct
b. Decreased Na reabsorption (and with it Cl) by inhibitng eNAC (epithelial Na channel) |
|
|
eNAC (Epithelial sodium channel)
a. Synonym b. Renal location c. Stimulated by d. Inhibited by |
a. Amiloride-sensitive sodium channel (ASSC)
b. Primary in principal cells of late distal tubule and collecting tubule c. Aldosterone d. Atrial natriuretic factor, Sodium channel blockers: amiloride, triamterene (Guyton) |
|
|
Hormonal control of tubular reabsorption - PTH
a. Site of action b. Effects (2) |
a. PT, thick ascending loop of Henle and early distal tubule
b. 1. ↓PO4 reabsorption (proximal tubule) 2. ↑Ca reabsorption (late distal tubule, maybe also loop of Henle. Also Mg reabsorption) (Guyton) |
|
|
Aquaporins - location & special characteristics
a. AQP-1 b. AQP-2 b. AQP-3 & 4 |
a. apical membrane of PT and thin descending limb of loop of Henle
b. apical membrane of late distal tubule, collecting tubules, and collecting ducts. Stimulated by ADH (short-term -> exocytosed of stored vesicles, long-term -> upregulation of gene transcription) b. Basolateral membrane (Wikipedia, Guyton) |
|
|
Renal clearance
a. What b. Formula |
a. The renal clearance of a substance is the volume of plasma that is completely cleared of the substance by the kidneys per unit time. (Can say that it refers to the volume of plasma that would be necessary to supply the amount of substance excreted in the urine per unit time)
b. Cs = (Us x V)\Ps Cs = Clearance rate of a substance s Ps = the plasma concentration of the substance Us = the urine concentration of the substance V = urine flow (Us x V) = urinary excretion (Guyton) |
|
|
Glomerular filtration rate (GFR)
a. Formual b. Which substance is used to measure it c. Which other substance can be used d. What method can be used to get a estimate of it |
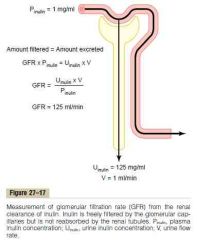
a. Image
b. Inulin c. Creatinine (a small amount is excreted, but this is cancelled out by the fact that measuring the plasma concentration give a slight overestimation) d. Plasma creatinine concentration |
|
|
Clearance ratio
|
Clearance ratio = Cs\Cinulin
(Guyton) |
|
|
Renal plasma flow
a. Substance used, why b. Formula |
a. p-aminohippuric acid (PAH), extraction ratio in healthy kidneys of 90%, due to extensive secretion in addition to filtration
b. RPF = Cpah\Epah Cpah = clearance of PAH (Cpah = (Upah x V)\Ppah) Epah = extraction ratio of PAH (arteriovenous concentration difference\arterial concentration) |
|
|
Excretion rate
|
Excretion rate = Us x V
Us = urine concentration of substance V = urine flow rate |
|
|
Renal blood flow
|
RBF = RPF\(1-hematocrit)
RPF = renal plasma flow (Guyton) |
|
|
Reabsorption & secretion rate
a. Formula for reabsorption rate b. Formula for secretion rate |
a. Reabsorption rate = filtered load - excretion rate = (GFR x Ps) - (Us x V)
b. Secretion rate = excretion rate - filtered load (Guyton) |
|
|
Osmolarity of urine
a. Lowest b. Highest |
a. 50 mOsm\L (1\6 of plasma)
b. 1200-1400 mOsm\L (Guyton) |
|
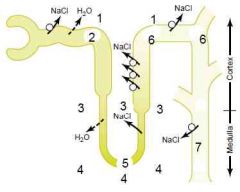
In the absence of ADH
|
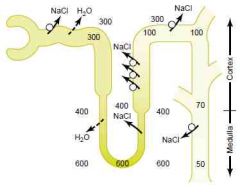
(Guyton)
|
|
|
Obligatory urine volume - formula with values
|
a. Ingested osmoles of solute each day\maximum osmolarity of urine
70kg man must excrete about 600 mOsm of solute each day, the maximum osmolarity is about 1200 mOsm\L -> 0.5L urine is the minimum (Seawater contains about 1200 mOsm\L (3-3.5% NaCl). Drinking 1L gives you 1200 mOsm to excrete. The kidney must also excrete other solutes, especially urea which contribute about 600 mOsm\L when the urine is maximally concentrated. Thus, the maximum concentratino of sodium chloride that can be excreted by the kidneys is 600 mOsm\L -> 2L of urine output for 1L of seawater) (Guyton) |
|
|
The four major factors that contribute to the hyperosmotic renal medullary interstitium
|
Factors causing buildup of solute concentration:
1. Active transport of sodium and co-transport of potassium chloride and others out of the thick portion of the ascending limp of the loop of Henle into the medullary interstitium (Can only create a gradient of 200 mOsm\L due to increased paracellular backflow of ions) 2. Active transport of ions from the medullary collecting ducts 3. Facilitated diffsuion of urea from the medullary collecting ducts 4. Diffusion of only small amounts of water from the medullary tubules into the medullary interstitium (Guyton) |
|
|
Counter-currents
a. What b. Examples (4) |
a. Counter-currents exist when fluids flow in opposite directions in parallel and adjacent tubes.
b. 1. The limbs of loop of Henle 2. The two limbs of the vasa recta 3. Dialysis 4. Artery & venae comites |
|
|
Countercurrent multiplier
|
The combined effect of solute transport in the ascending limb and osmosis in the thin descending limb of the loop of Henle to create the hyperosmotic medullary interstitium
(The solute transport in the TAL of Henle could not doe it on its own because it can only produce a concentration gradient of 200 mOsm\L due to paracellular back-leakage of ions) (Guyton) |
|
|
What is the role of the distal tubule and collecting tubules and ducts in maintaining the hyperosmotic renal medullary interstitium
|
a. Solute pumping in the absence of ADH (eNAC + Na-K-ATPase)
b. In the presence of ADH, most of the water is reabsorbed into the cortex rather than into the renal medulla. And from here it is swept away by rapidly flowing "efferent" peritubular capillaries (Guyton) |
|
|
Urea's contribution to hyperosmotic renal medullary interstitium and thus to a concentrated urine
a. Quantitative contribution to the renal medullary interstitium when the kidney is forming a maximally concentrated urine b. How |
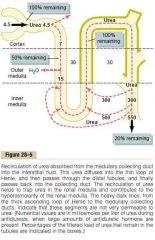
a. About 40-50% of the osmolarity of the renal medullary interstitium (500-600 mOsm\L) when the kidney is formign a maximally concentrated urine
b. 1. Urea is impermeable to the TAL, distal tubule, collecting tubule, and cortical collecting duct 2. ↑ADH -> water reabsorption starting from late distal tubule -> ↑concentration of urea 3. Urea is permeable to the medullary connecting duct -> diffuse into medullary interstitium along with water thus maintaining a high concentration in the urine while it is being reabsorbed (facilitated by UT(Urea transporter)-A1 activated by ADH) 4. Diffuse back into thin limbs of loop of Henle -> recirculation (can recirculate several times before it gets excreted, each time around it contributes to a higher concentration of urea) (Malnutrition is assoicated with a low urea concentration in the medullary interstitium and considerable impairment of urine concentrating ability) (Guyton) |
|
|
Is the osmolarity of the renal medullary interstitium dependent on ADH? How? Why?
|
Yes.
When ADH levels are high, more urea is being reabsorbed into the medullary interstitium from the collecting ducts (which accounts for 40-50% of the osmolarity) (Guyton) |
|
|
Free-water clearance
a. What b. Formula |
a. The rate at which solute-free water is excreted by the kidneys
b. Ch2o = V - Cosm V = urinary flow rate Cosm = osmolar clearance (total clearance of solutes) (Guyton) |
|
|
Central diabetes insipidus - treatment
|
Give desmopressin, which is a synthetic analog that is selective for V2 receptors
(Guyton) |
|
|
Nephrogenic diabates insipidus - causes (3)
|
a. End-stage renal disease (damage to > 80% of nephrons)
b. Loop diuretics c. Drugs - lithlium (against manic-depressive disorders) and tetracycline (Guyton) |
|
|
How can plasma osmolarity be estimated from plasma sodium concentration?
|
Posm = 2.1 x plasma sodium concentration
(Sodium and its associated anions account for about 94% of the solute in the extracellular compartment) (Guyton) |
|
|
Osmoreceptor-ADH feedback system
a. Where is the osmoreceptors located? b. How are they activated? c. What is the next step? |
a. In the anteroventral region of the third ventrcle called the AV3V region - close to both the subfornical region (in the upper part) and the organum vasculosum of lamina terminalis (OVLT) (lower). (Both these regions have vascular supplies that lack the typical blood-brain barrier -> ions and other solutes can cross between the blood and the local interstitial space)
b. In the presence of hyperosmolarity they shrink, which cause them to depolarize c. They send nerve signals to to the median preoptic nucleus -> magnocellular neurons in the supraoptic nucleus (5\6 of ADH production) and the paraventricular nucleus (1\6 of ADH production) (Also to blood pressure control centers in the medulla and to centers responsible for thirst and sodium appetite) (Guyton) |
|
|
Which other factors can control ADH release?
|
1. The arterial baroreceptor reflexes
2. The cardiopulmonary reflexes (1 &2: In response to hypotension or hypovolemia (after >10% loss)) 3. Nausea 4. Hypoxia (Guyton) |
|
|
Which drugs can influence the ADH secretion
a. Stimulate it (3)y of b. Inhibit it (3) |
a.
1. Morphine 2. Nicotine 3. Cyclophosphamide b. 1. Alcohol 2. Clonidine 3. Haloperidol (dopamine blocker) (Guyton) |
|
|
Thirst center
a. Where b. Stimuli for thirst (4) |
a. in The AV3V region in the anteroventral wall of the third ventricle, especially a small nucleus anterolateral to the preoptic nucleus
(Anterolaterally to the preoptic nucleus is a area that cause immmedate drinking when stimulated. Organum vasculsom of lamina terminalis (OVLT) is immediately beneath the ventricular surface and can be involved in increasing thirst in response to increased osmolarity of the CSF) b. 1. ↑Osmolarity of ECF & CSF (when >2 mOsm\L) 2. Hypovolemia & hypotension (<- baroreceptor & cardiopulmonary reflexes) 3. ATII (act on subfornical organ and OVLT that is outside the BBB) 4. Dryness of mucus membrane of mouth and esophagus (Gastric distention may partially alleviate thirst) |
|
|
Sodium intake
a. Average sodium intake in the industrialized world b. Humans can survive and function normally on which value? |
a. 100-200 mM
b. 10-20 mM (Guyton) |
|
|
Salt-appetite
a. Centers b. Stimuli |
a. AV3V region, closely related to thirst
b. 1. Hyponatremia 2. Hypovolemia & hypotension (Guyton) |
|
|
Regulation of internal potassium distribution (7)
|
1. Insulin (-> influx) (diabetics have higher rise in K after a meal)
2. Aldosterone (->influx) (Conn's -> hypokalemia, Addison's -> hyperkalemia) 3. B2 stimulation (-> influx) (epinephrine -> hypokalemia, beta-blocker -> hyperkalemia) 4. Acid-base (alklaosis -> influx (hypokalemia), acidosis -> efflux (hyperkalemia). incompletely understood, but one effect of decreased pH is to reduce Na-K-ATPase) 5. Cell lysis (crush injury, rhabdomyolsis, tumor lysis syndrome (post-chemo, lymphomas) 6. Strenuous exercise (by relasing K from skeletal muscle)(mild, can be higher in persons on beta-blockers or individuals with insulin deficiency) 7. Extracellular fluid osmolarity (↑-> efflux: due to efflux of water because of osmosis with subsequent ↑[K] intracellularly. Diabetes mellitus -> ↑ECF osmolarity -> efflux of K (hyperkalemia)) (Guyton) |
|
|
Factors that regulate potassium secretion (3)
|
1. Plasma potassium concentration (↑Na-K-ATPase of principal cells, increase the K gradient from renal interstitium to principal cells -> less backleakage, stimulate aldosterone)
2. Aldosterone stimulate potassium secretion (Principal cells: ↑basolateral Na-K-ATPase, ↑luminal permeability of K) 3. Distal tubular flow rate -> ↑ (When K is secreted into the tubular fluid, its luminal concentration increase and thereby reduce the driving force for K diffusion. ↑tubular flow rate -> ↑flushing of luminal K -> less reduction of the driving force of K diffusion)(Occurs with volume expansion, high sodium intake) 4. Acute acidosis -> ↓, Acute alkaosis -> ↑ (Acidosis inhibit basolateral Na-K-ATPase) (Chronic acidosis leads to ↑K loss. Inhibit basolateral Na-K-ATPase also in proximal tubule -> ↓NaCl & water reabsorption -> ↑distal tubular flow rate -> ↑K excretion) (Guyton) |
|
|
Hypocalcemia
a. Mechanism b. Symptoms (2) |
a. Ca inhibit neuronal membrane permability of Na by acting on the channels (cause hyperpolarization in microenvironment of Na channels). -> Hypocalcemia -> less inhibition of Na permeability -> easy initiation of action potentials
b. 1. Tetanus (at 35% reduction of plasma levels) a. Carpopedal spasm 2. Seizure (Lethal at 60% reduction of plasma levels) |
|
|
Hypercalcemia
a. Mechanism b. Symptoms |
a. Ca inhibit neuronal membrane permability of Na by acting on the channels (cause hyperpolarization in microenvironment of Na channels). -> Hypercalcemia -> ↑inhibition of Na channels -> difficult to initiate action potentials)
b. 1. CNS depression (sluggish, decreased reflexes) 2. Heart - decrease QT interval 3. Constipation (depressed contarctility of the muscle walls of the gastrointestianl tract) |
|
|
Calcium
a. Forms % proportions of calcium in the plasma b. How is the pH related |
a.
1. Free - 50% 2. Bound to plasma proteins - 40% 3. Complexed with anions (phosphate, citrate) - 10% b. In acidosis H+ displace Ca2+ from plasma proteins causing hypercalcemia while in allkalosis Ca2+ has more binding spots on plasma proteins causing hypocalcemia (Guyton) |
|
|
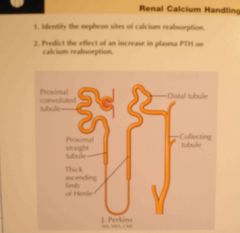
Reabsorption of Ca and Na - percentage in
a. Proximal tubule b. Loop of Henle c. Distal and collecting tubules |
a. 65%
b. 25-30% c. 4-9% (Guyton) |
|
|
Calcium excretion
a. % by kidneys b. % by feces |
a. 10%
b. 90% (Guyton) |
|
|
Renal Ca excretion - regulating factors (6)
|
1. PTH - decrease Ca excretion (TAL, distal tubule)
2. -Volemia. ie. Hypervolemia -> ↑Ca excretion (Ca reabsorption parallels Na in the proximal tubule. Thus, hypervolemia\hypertension -> ↓proximal Na, H2O, Ca reabsorption) 3. -Tension (hypertension -> ↑Ca excretion) 4. Plasma phosphate - hyperphoshatemia increase Ca reabsorption by stimulating PTH 5. Metabolic Acid-base: ie acidosis -> ↑reabsorption (by inhibiting Na-K-ATPase and luminal-cellular Na gradient) 6. Vitamin D -> ↑Ca reabsorption (Guyton) |
|
|
Magnesium
a. % in bone, % extracellularly b. % reabsorption in proximal tubule, loop of Henle c. Factors leading to increased excretion (4) |
a. > 50% in bone, 1% in extracellular compartment (Of this over 50% is bound to plasma proteins)
b. Proximal tubule: 25%, Loop of henle: 65% (10-15% is excreted) c. 1. Hypermagnesemia 2. Hypercalcemia 3. Hypervolemia 4. Increased PTH (Mechanisms not completely understood) (Guyton) |
|
|
ATII
a. What is the mechanism for chronic blood-pressure lowering effects in hypertensive patients of the ACE-I or ATII receptor antagonists |
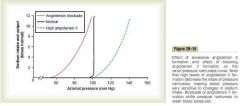
a. High ATII decrease the slope of pressure natriuresis, making blood pressure very sensitve to changes in sodium intake and requiring much greater increase in arterial pressure to increase sodium excretion and main sodium balance. blockade by either of the two agents shifts pressure natriuresis to lower blood pressure.
(Guyton) |
|
|
pH
a. Arterial value b. Venous value c. Upper and lower limit compatible with life fore more than a few hours d. Range in urine e. Intracellular value |
a. 7.4 (40 nM)
b. 7.35 (45 nM) c. 6.8-8 d. 4-5-8 e. 6-7.4 (The pH of the gastric HCl secretions by parietal cells is 0.8) (Guyton) |
|
|
What is the relationship with pCO2 and [CO2]?
|
CO2 = pCO2 x solubility coefficient (0.03 mM\mmHg)
(Guyton) |
|
|
Henderson-Hasselbalch equaton for the bicarbonate buffer system
|
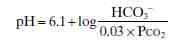
|
|
|
Draw the bicarbonate buffer system titration curve, showing the pH on X of extracellular fluid when the percentages of buffer in the form of bicarbonate and CO2 (H2CO3) are altered by adding acid or base respectively
|
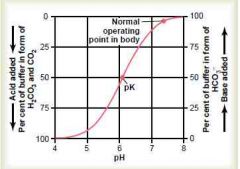
|
|
|
Phosphate buffer
a. Components b. Important in which two locations c. pK |
a. H2PO^4- and HPO4^2-
b. 1. Intracellular (higher concentration here and the pH is closer to its pK) 2. In renal tubular fluid (because it is greatly concentrated here and the pH here is closer to its pK) c. 6.8 (Closer to the pH of extracellular fluid than the pK of the bicarbonate buffer system, but the extracellular concentration is only 8% of that of the bicarbonate buffer system) (Guyton) |
|
|
Intracellular pH in relation to extracellular pH
a. How are they related b. Why are proteins important buffers intracellularly? |
a. Change in proportion to EC changes, but not to the same extent. H and HCO3 can only diffuse through the cell membrane slowly (except in RBCs), CO2 however can rapidly diffuse
b. High concentration, pK close to 7.4. (Approximately 60-70% of the total chemical buffering of the body fluids is inside the cells, and most of this results from the intracellular proteins, but this system is slow to buffer extracellular changes (several hours)) (Guyton) |
|
|
Change in extracellular fluid pH caused by change in alveolar ventilation
a. Increasing rate of alveolar ventilation by 2x b. Decreasing rate of alveolar ventilation to 1\4 |
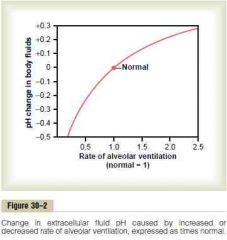
a. Increase pH by about 0.2 (-> 7.8)
b. Decrease pH by about 0.45 (-> 6.95) (Guyton) |
|
|
Effect of pH on avelolar ventilation rate
a. If pH is decreased to 7 |
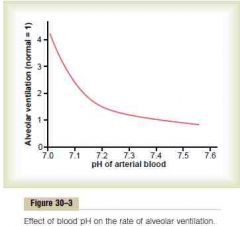
4 x alveolar ventilation rate
(The change in alveolar ventilation rate per unit pH change is much higher at reduced levels of pH than at increased levels of pH. This is because the alveolar ventilation is inhibited from being excessively depressed by simultaneous hypoxia) (Guyton) |
|
|
Efficiency of respiratory control to compensate for an alteration of pH outside the respiratory system
|
The respiratory mechanism for controlling H+ concentration has an effectiveness between 50-75% (feedback gain of 1-3)
(ie pH drops to 7 -> can only be compensated to 7.2-7.3) (Takes 3-12 minutes) (Guyton) |
|
|
The kidneys regulate extracellular pH by three fundamental mechanisms
|
1. Secretion of H
2. Reabsorption of bicarbonate 3. Production of new bicarbonate (Guyton) |
|
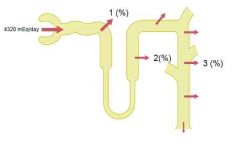
|
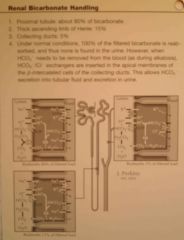
Reabsorption of bicarbonate (and secretion of H) occurs to some degree in all parts beside the thin descending and ascending limbs of loop of Henle. For each bicarbonate reabsorbed, one H must be secreted.
(Guyton) |
|
|
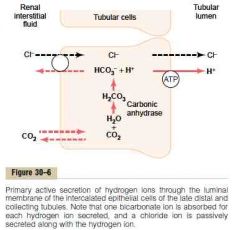
Secretion of H and reabsorption of bicarbonate in the proximal tubule, TAL and early distal tubule
a. Mechanism b. How does HCO3 exit the basolateral membrane |
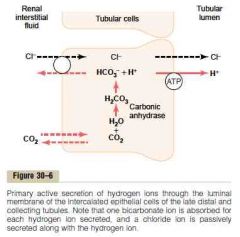
a.
1. CO2 (from inward diffusion or cell's metabolism) combine with H2O to form H2CO3, catalyzed by carbonic anhydrase. H2CO3 dissociate to H and HCO3 (The HCO3 generated diffuse into the peritubular capillary. The net result is that for every H secreted into the tubular lumen, an HCO3 enters the blood) 2. The H is secreted into the lumen by secondary active Na-H counter-transport driven by basolateral Na-K-ATPase 3. H combines with filtered HCO3 to form H2CO3. This dissociates to CO2 and H2O luminal carbonic anhydrase (type IV) (Drugs that inhibit carbonic anhydrase (e.g., acetazolamide) interfere with proximal reabsorption of NaHCO3 and induce a bicarbonate (osmotic) diuresis.) 4. CO2 diffuses into the cell -> new cycle (This sequence of events is necessary because the luminal membrane has a very low permeability to HCO3) b. HCO3 exit the basolateral membrane by 1. Na-HCO3 co-transporter 2. Cl-HCO3 exchanger (Guyton) |
|
|
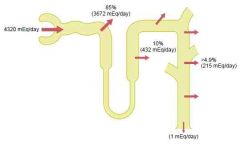
Bicarbonate & H under normal conditions
a. Rate of tubular secretion of H? b. Rate of filtration of HCO3? c. Excess of HCO3 over H in the renal tubules as in alkalosis cause? d. Excess of H over HCO3 in the renal tubules as in acidosis cause? |
a. 4400 mmol\day
b. 4320 mmol\day c. The excess HCO3 cannot be reabsorbed -> left in renal tubule -> excreted d. 1. Cause complete reabsorption of bicarbonate 2. Excess H is buffered in the tubules by phosphate and ammonia -> excreted as their salts (ie NaH2PO4) 3. Formation of new bicarbonate (whever an H secreted into the tubular lumen combines with a buffer other than HCO3, the net effect is addition of a new HCO3 to the blood) (Ammonia is quantitatively far more important, phosphate normally only have 30-40 mmol\day for buffering) (Guyton) |
|
|
Mechanism of H secretion in late distal tubule and collecting tubules
a. In which cells b. Mechanism c. % of total renal H secretion |
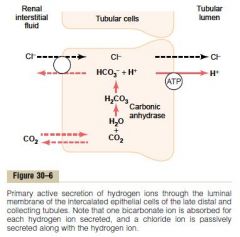
a. Intercalated cells
b. 1. Dissolved CO2 (<- metabolism, inward diffusion) combine with H2O to form H2CO3, catalyzed by intracellular carbonic anhydrase. Then it dissociates and bicarbonate is reabsorbed into the peritubular capillaries 2. H is secreted by H-ATPase in the luminal membrane (Thus here as well is one bicarbonate reabsorbed for every H secreted) c. 5% (In the Na-H-counter-transport H can only be increased 3-4 fold in the renal tubule (pH 6.7), while by this active pump, H concentration in the renal tubule can increase by 900-fold (pH 4.5)) (Guyton) |
|
|
Why must H be combined with phosphate and ammonia buffers when it is excreted in excess of bicarbonate?
|
Because the minimal urine pH is 4.5. This corresponds to a very low concentration of H (0.03 mM), and it is not sufficient to excrete the nonvolatile acids (80 mmol)
(By combining with phosphate and ammonia buffers, as much as 500 mmol\day can be excreted) (Guyton) |
|
|
Ammonia renal tubular buffer system
a. From where b. Mechanism in collecting tubule c. Is this affected by acidosis or alkalosis? |
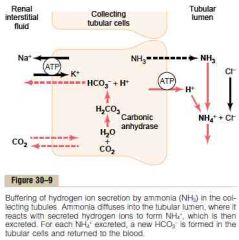
a. Metabolism of AA in liver (mostly) -> glutamine -> 2 bicarbonate & 2 ammonium
b. Mechanism in collecting tubule 1. H is secreted into the tubular lumen by H-ATPase, here it combines with ammonia to form ammonium 2. The collecting tubule are permeable to ammonia, which diffuse into the tubular lumen. However its much less permeable to ammonium, thus trapping it in the renal tubule (For each ammonium excreted, a new bicarbonate is generated and added to the blood) c. Acidosis stimulate renal glutamine metabolism (and therefore increase the formation of NH4 and new bicarbonate to be used in H buffering), a decrease has the opposite effect (The ammonia buffer system accounts for about 50% of the acid excreted and 50% of the new bicarbonate generated by the kidneys) (Guyton) |
|
|
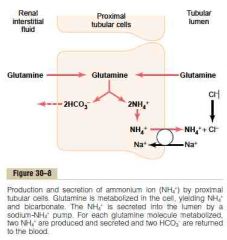
Ammonia renal tubular buffer system
a. From where b. Mechanism in proximal tubule, TAL, and early distal tubule c. Is this affected by acidosis or alkalosis? |
a. Metabolism of AA in liver (mostly) -> entry into renal tubular cell -> glutamine -> 2 bicarbonate & 2 ammonium
b. Mechanism in proximal tubule, TAL, and early distal tubule 1. The bicarbonate is transported over the basolateral membrane by HCO3-Cl-exchanger and Na-HCO3 co-transport 2. Ammonium is secreted into the tubular lumen by sodium-ammonim counter-transporter (Thus, for each molecule of glutamine metabolized here, two ammoinum are secreted into the urine and two NEW bicarbonates are reabsorbed into the blood) c. Acidosis stimulate renal glutamine metabolism (and therefore increase the formation of NH4 and new bicarbonate to be used in H buffering), a decrease has the opposite effect (The ammonia buffer system accounts for about 50% of the acid excreted and 50% of the new bicarbonate generated by the kidneys) (Guyton) |
|
|
The Reticular Formation:
|
Network of neurons in the medulla and other parts of the brainstem. The descending portion controls motor areas of the spinal cord; ascending portion sends output to much of the cerebral cortex, selectively increasing arousal and attention.
|
|
|
Net acid excretion - formula
|
Net acid excretion = Ammonium excretion + urinary titratable acid - bicarbonate excretion
Titratable acid: phosphate and other organic buffers (titrate urine to pH 7.4 by strong buffer, don't affect ammonium because its pK is 9.2) (Excretion = urinary concentration of substance x urine flow) (Subtract bicarbonate excretion because the loss of bicarbonate is the same as the addition of H to the blood) (The net acid excretion also equals the rate of net bicarbonate addition to the blood) (In alkalosis there is a negative net acid excretion because NH4 and titratable acid excretion drop almost to zero while bicarbonate excretion increase) (Guyton) |
|
|
Factors that regulate H secretion and bicarbonate reabsorption by the renal tubules - examplify by how it increase H secretion and HCO3 reabsorption (6)
|
1. pCO2 - ↑->↑H secretion & ↑HCO3 reabsorption (By CO2 diffusing into cells and increasing intracellular H concentration, thus creating more substrate and higher gradient)
2. H, HCO3. ↑H & ↓HCO3 -> ↑H secretion & ↑HCO3 reabsorption (more substrate and higher gradient for H) 3. Extracellular fluid volume - ↓ECV -> ↑H secretion & HCO3 reabsorption (Indirectly by increasing sodium reabsorption, which increase Na-H counter-transporter) 4. ATII - ↑ -> ↑H secretion & HCO3 reabsorption (directly stimulate Na-H counter-transporter) 5. Aldosterone - ↑ -> ↑secretion & HCO3 reabsorption (stimulate H-ATPase of intercalated cells of cortical collecting tubules and late distal tubules) 6. Potassium - hypokalemia -> ↑H secretion and HCO3 reabsorption (Hypokalemia tend to increase intracellular [H] in the renal tubular cells -> stimulate H secretion) (Guyton) |
|
|
Metabolic acidosis - causes (4)
|
1. Failure of the kidneys to excrete metabolic acids normally found in the body (renal tubular acidosis (ie Addison's disease, chronic renal failure, Fanconi syndrome) - renal tubular cells cant excrete H or reabsorb HCO3)
2. Formation of excess quantities of metabolic acids in the body (DM (acetoacetic acid)) 3. Addition of metabolic acids to the body by ingestion or infusion of acids (aspirin, methanol) 4. Loss of base from the body fluid (most common cause. Ie diarrhea, vomiting (from intestine)) |
|
|
Metabolic alkalosis - causes (4)
|
1. Diuretics (except carbonic anhydrase inhibitors) (-> ↑distal flow -> ↑reabsorption of Na -> H secretion is coupled to Na reabsorption)
2. Excess aldosterone (promote extensive reabsorption of Na from the distal and collecting tubules, stimulate H-ATPase in intercalated cells) 3. Vomiting of gastric contents alone (neonates with pyloric obstruction) 4. Ingestion of alkaline drugs (NaHCO3 <- for gastritis or peptic ulcer) (Guyton) (Guyton) |
|
|
Why is sodium gluconate or sodium lactate given IV for metabolic acidosis?
|
1. The sodium (Na+) ion combines with bicarbonate ion produced from carbon dioxide of the body and thus retains bicarbonate to combat metabolic acidosis (bicarbonate deficiency).
2. The lactate anion is in equilibrium with pyruvate and has an alkalizing effect resulting from simultaneous removal by the liver of lactate and hydrogen ions as it makes glycogen (assuming the metabolic acidosis is not related to high lactate) (Dailymed) |
|
|
Lactate - why does it not directly cause acidosis?
|
This is because lactate itself is not capable of releasing a proton,[7] and, second, the acidic form of lactate, lactic acid, "is not produced in muscle".[8]
(Analysis of the glycolytic pathway in humans indicates that there are not enough hydrogen ions present in the glycolytic intermediates to produce lactic or any other acid.) When ATP is hydrolysed, a hydrogen ion is released. ATP-derived hydrogen ions are responsible primarily for the decrease in pH. During intense exercise, aerobic metabolism cannot produce ATP quickly enough to supply the demands of the muscle. As a result, anaerobic metabolism becomes the dominant energy-producing pathway, as it can form ATP at high rates. Due to the large amounts of ATP being produced and hydrolysed in a short period of time, the buffering systems of the tissues are overcome, causing pH to fall and creating a state of acidosis (^ a b c Robergs, RA; Ghiasvand, F; Parker, D (2004). "Biochemistry of exercise-induced metabolic acidosis". Am J Physiol Regul Integr Comp Physiol 287 (3): R502–R516. doi:10.1152/ajpregu.00114.2004. PMID 15308499. ^ a b Lindinger, M. I. (2004). "Applying physicochemical principles to skeletal muscle acid-base status". Am J Physiol Regul Integr Comp Physiol 289 (3): R890–94. doi:10.1152/ajpregu.00225.2005. ^ Siggaard-Andersen, O.; G�thgen, I. H. (1995). "Oxygen and acid-base parameters of arterial and mixed venous blood, relevant versus redundant". Acta Anaesthesiologica Scandinavica 39: 21. doi:10.1111/j.1399-6576.1995.tb04325.x.) |
|
|
Treatment of alkalosis
|
Ammonium chloride by mouth
(ammonium is converted in the liver into urea. This reaction liberates HCl, which immediately reacts with the buffers of the body fluids to produce an more acidic state) (Guyton) |
|
|
Time for full compensatory response in acid-base disorders
a. For ventilatory compensations in metabolic disorders b. For metabolic compensations in ventilatory disorders |
a. 6-12 hours
b. 3-5 days (Guyton) |
|
|
Plama anion gap
a. What b. Normal value c. Use |
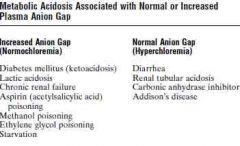
a. Clinical concept
Plasma anion gap = [Na] - [HCO3] - [Cl] b. 8-16 mM c. To differentiate different types of metabolic acidosis (Metabolic acidosis caused by excess nonvolatile acids (except HCl), such as lactic acid or ketoacids, is associated with an increased plasma anion gap because the fall in bicarbonate is not matched by an equal increase in Cl-) (Guyton) |
|
|
Loop diuretics
a. Which (3) b. Block Na-2Cl-K co-transporter in TAL, which effects has this (2)? c. How many percent of the glomerular filtrate may be delivered to the urine? |
a. Furosemide, Bumetanide, Ethacrynic acid
b. 1. They greatly increase the quantities of solutes delivered to the distal parts of the nephrons, and these act as osmotic agents 2. They disrupt the countercurrent multiplier (This impairs the kidneys ability to both concentrate and dilute urine) c. 20-30% of the glomerular filtrate may be delivered to the urine (Causing, under acute conditions, urine output to be as great as 25 times normal for a few minutes) (Guyton) |
|
|
Thiazide diuretics
a. Which (2) b. Mechanism c. How many % of the glomerular filtrate can they cause to pass into the urine |
a. Hydrochlorothiazide, chlorthalidone
b. Inhibit Na-Cl co-transport in luminal membarne of early distal tubules c. Can cause 5-10% of the glomerular filtrate to pass into the urine (Guyton) |
|
|
Carbonic anhydrase inhbitors
a. Which (1) b. Mechanism c. Adverse effect |
a. Azetazolamide
b. Carbonic anhydrase is abundant in the proximal tubule. Inhibit H secretion and HCO3 reabsorption. Na reabsorption is linked to H via Na-H counter-transport, thus decreasing reabsorption of sodium. Cause sodium to remain in the tubule and act as an diuretic c. Acidosis due to loss of bicarbonate (Guyton) |
|
|
Intrarenal acute renal failure - causes
a. Small vessel and\or glomerular injury (4) b. Tubular epithelial injury (tubular necrosis) (2) c. Renal interstitial injury (2) |
a. Small vessel and\or glomerular injury
1. Vasculitis (polyarteritis nodosa) 2. Cholesterol emboli 3. Malignant hypertension (severe hypertension, cause necrosis of renal arteriolar walls) 4. Acute glomerulonephritis b. Tubular epithelial injury (tubular necrosis) 1. Acute tubular necrosis due to ischemia 2. Acute tubular necrosis due to toxins: heavy metals, ethylene glycol, insecticides, poison mushrooms c. Renal interstitial injury 1. Acute pyelonephritis 2. Acute allergic interstitial nephritis (Guyton) |
|
|
Prerenal acute renal failure - causes
a. Intravascular volume depletion (3) b. Cardiac failure (2) c. Peripheral vasodilation and resultant hypotension (3) c. Primary renal hemodynamic abnormalities (2) |
a. Intravascular volume depletion
1. Hemorrhage (trauma, surgery, postpartum, GI) 2. Diarrhea or vomiting 3. Burns b. Cardiac failure - MI, valvular c. Peripheral vasodilation and resultant hypotension 1. Anaphylactic shock 2. Anesthesia 3. Sepsis, severe infections c. Primary renal hemodynamic abnormalities 1. Renal artery stenosis 2. Thrombosis or embolism of renal artery or vein (Decrased blood supply) (Can last below 20% of renal blood flow for a few hours before permanent danger occur) (Guyton) |
|
|
Acute glomerulonephritis
a. Mechanism b. Prognosis |
a. (for 95% of patients)
1. Group A beta streptococcal infection (throat, tonsillitis, skin) -> (few weeks) 2. Antigen-antibody react with each other to form an insoluble immune complex which becomes trapped in the glomeruli, especially basement memebrane 3. -> reactive hyperproliferation (especially of mesangial cells), chemotaxis of large amount of white blood cells which also becomes entrapped and potentiates immune reaction 4. Glomeruli can become blocked or excessively permeable (immune reaction) and leak cells and protein b. The acute inflammation of the glomeruli usually subside in 2 weeks, and in most patients the kidneys return to almost normal function (within the next weeks and months) (Guyton) |
|
|
How does acute tubular necrosis (ischemic or toxic) cause decreased urine output?
|
The dead tubular cells slough off and "plug" the tubules
(Guyton) |
|
|
Acute renal failure - important physiological effects
|
1. Hyperkalemia (8mM can be fatal)
2. Metabolic acidosis (can't excrete H) (The patients will die in 1-2 weeks if not kidney function is restored or dialysis treatment is used) (Guyton) |
|
|
Chronic renal failure - serious clinical symptoms usually don't arise before there only remains .... % of functional nephrons
|
20-25%
(Death usually ensues when the number is down to 5-10%) (Guyton) |
|
|
Chronic renal failure - causes (8)
|
1. Metabolic disorders (DM, obesity, amyloidosis)
2. Hypertension 3. Infection (tuberculosis, pyelonephritis) 4. Congenital disorders (renal hypoplasia, polycystic disease) 5. Primary tubular disorder (nephrotoxins) 6. Immunologic disorders (lupus erythematosus, glomerulonephritis, polyarteritis nodosa) 7. Renal vascular disorders (atherosclerosis (larger arteries), nephrosclerosis-hypertension (smaller arteries, arterioles, glomeruli) 8. Urinary tract obstruction (renal caliculi, hypertrophy of prostate, urethral constriction) (Hypertension (26%) & DM (44%) account for 70% of cases) (Guyton) |
|
|
Vicious circle of chronic renal failure
|
1. Primary kidney disease ->
2. ↓Nephron number -> 3. Adaptive changes: a. Hypertrophy and vasodilation of surviving nephrons b. ↑arterial pressure -> 4. ↑Glomerular pressure and\or filtration -> 5. Glomerular sclerosis (replacement with connective tissue) -> 6. ↓Nephron number (Can be slowed down by giving ACE inhibitor or antagonist to lower glomeruler hydrostatic pressure and arterial pressure) (Guyton) |
|
|
How many % of the risk of developing essential hypertension can be attributed to excessive weight gain?
|
65-75%
(Guyton) |
|
|
Benign nephrosclerosis - mechanism
|
Affect smaller interlobular arteries and afferent arterioles, since these don't have collateral circulation, their supplying nephron is non-functional as well
Begin with leakage of plasma through the intimal membrane -> fibrinoid deposits in medial layers -> progressive thickening (Most common kidney disease, Found in 70% of postmortem examinations of people over 60 years) (Progressed by other disorders. Hypertension combined with benign nephrosclerosis can lead to rapidly progressing malignant nephrosclerosis) (Guyton) |
|
|
Renal interstitial injury caused by bacterial infection is called? What is the most common causative agent?
|
Pyelonephritis
Escherichia coli from fecal contamination (Guyton) |
|
|
Two risk factors for cystitis
|
1. The inability of the bladder to empty completely
2. The existence of obstruction of urine outflow (With impaired ability to flush bacteria from the bladder, the bacteria multiply and the bladder becomes inflamed) (Combined with vesicoureteral reflux the risk of pyelonephritis is greatly increased) (Guyton) |
|
|
Nephrotic syndrome
a. What b. Causes (3) |
a. Characterized by loss of large quantities of plasma proteins into the urine due to increased permeability of the glomerular membrane
b. 1. Chronic glomerulonephritis (greatly increase glomerular membrane permeability) 2. Amyloidosis (Deposition of an abnormal proteinoid substance in the walls of the blood vessels, and seriously damages the basement membrane of the glomeruli) 3. Minimal change nephrotic syndrome (No abnormality on light microscope, loss of negative charge on basement membrane? antibody-mediated? more often in children 2-6 years) (Guyton |
|
|
Number of nephrons
|
2 000 000
(Guyton) |
|
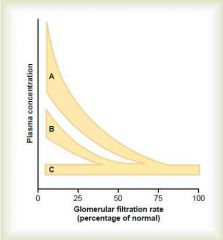
ABC?
|
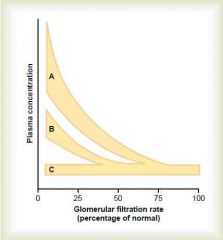
A = creatinine, urea (substances that depend largely on glomerular filtration for excretion)
B = phosphate, urate, H (most are secreted) C = Na, Cl (Guyton) |
|
|
Isosthenuria
a. What b. Cause in chronic renal failure |
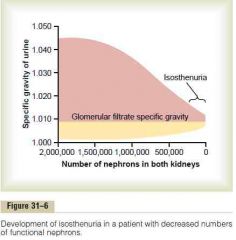
a. Inability of the kidney to concentrate or dilute urine
b. 1. The rapid flow of tubular fluid through the collecting ducts prevents adequate water reabsorption 2. The rapid flow though both the loop of Henle and the collecting ducts prevents the countercurrent mechanism 3. High solute flow and rapid flow prevent dilution (Guyton) |
|
|
a. Effects of renal failure on the body fluids (4)
b. Name of this total condition? c. 2 Other effects |
a. 1. Generalized edema from water and salt retention
2. Acidosis (resulting from failure of the kidneys to rid the body of normal acidic products and reabsorption of bicarbonate) 3. High concentration of nonprotein nitrogens (azotemia) - urea, creatinine, urate 4. High concentrations of other substances - phenols, sulfates, phosphates, potassium, guanidine bases b. Uremia c. 1. Anemia 2. Osteomalacia (vitamin D, ↑phosphate) (↑phosphate -> ↑binding to serum Ca -> ↓free serum Ca -> ↑PTH (secondary parathyroidism)) (Guyton) |
|
|
Specific tubular disorders - caused by congenital defects or damage by toxins or ischemia (6)
|
1. Renal glycosuria (benign, ruled out with DM)
2. Aminoaciduria (essential cystinuria (-> renal stone), simple glycinuria, generalized aminoaciduria) 3. Renal hypophosphatemia (relatively benign, rickets refractory to vitamin D) 4. Renal tubular acidosis (inability to secrete H) 5. Nephrogenic diabetes insipidus 6. Fanconi's syndrome (Guyton) |
|
|
Fanconi's syndrome
a. What b. Causes (3) |
a. A generalized reabsorptive defect of the renal tubules - virtually all amino acids, glucose, phosphate
(In severe cases there can also be failure to reabsorb NaHCO3 -> metabolic acidosis, increased excretion of K and Ca, nephrogenic diabetes insipidus) b. 1. Hereditary defects in cell transport mechanisms 2. Toxins 3. Ischemic injury (The proximal tubular cells are especially affected when its caused by tubular injury, because these cells reabsorb and secrete many of the drugs and toxins that cause damage) (Guyton) |
|
|
ATII constricts renal arterie, arterioles, and?
|
Glomerular mesangial cells (This helps to reduce GFR further)
(Netter) |
|
|
Describe the role of intrarenal prostaglandins on the GFR
|
Intrarenal prostaglandins - PGE2 and PGI2 are vasodilators
(Primarily serve to oppose the vasoconstrictor actions of ATII on arterioles and mesangial cells) (Netter) |
|
|
Intraglomerular mesangial cells
a. What type of cell, location b. Functions (4) |
a. Modified pericytes, located among the glomerular capillaries within the basement membraneEx
b. 1. Structural support 2. Can contract in response to autacoids -> decrease blood flow to glomerular capillaries and by being connected to the basement membrane cause decrease in its surface area 3. Contributors to extracellular matrix (fibronectin, IV collagen, laminin) 4. Phagocytosis (glomerular basal lamina components, immunoglobulins. Phagocytic cell derived from smooth muscle and not monocytes) (Wikipedia) |
|
|
Extraglomerular mesangial cells
a. Location b. Function |
a. Outside the glomerulus near the vascular pole and macula densa
b. Component of the juxtaglomerular apparatus, uncertain function, associated with EPO? (Wikipedia) |
|
|
Identify the two populations of nephrons and describe their different structural and functional characteristics
|
Cortical (80%) and juxtamedullary nephrons
Cortical nephrons's loop of henle extends into the outer zone of the medulla, while the juxtamedullary nephron extends into the inner zone The juxtamedullary nephron have longer proximal convoluted tubules which allow greater proximal tubule bulk reabsorption. The long loopf of Henle is responsible for the countercurrent multipler (Netter) |
|
|
|
Located in the collecting duct.
(Wikipedia) |
|

|

Located in the collecting duct.
(Wikipedia) |
|
|
Band 3\Anion exchanger 1 (AE1)
a. What? b. Where? |
a. Counter-transporter of Cl-HCO3
b. Erythrocytes, basolateral surface of alpha-intercalated cells of collecting duct (acid-secreting) (Wikipedia) |
|
|

(Netter)
|
|
|
(Netter)
|
|
|

(Netter)
|
|
|

(Netter)
|
|
|
The nephron
a. Blood-primary filtration barrier b. Mesangial cells c. Number of nephrons in each kidney d. Total surface area of the glomerular capillary endothelium e. Total surface area of the tubules, total surface area of the renal capillaries |
a. Blood-primary filtration barrier
1. Capillary endothelium I. Fenestrated - 70-90 nm pores 2. Basal lamina 3. Podocyte pseudopodia I. Filtration slits between pseudopodia - 25 nm c. 1.3 million d. 0.8 m2 e. 12 m2 (Ganong) b. Mesangial cells I. Stellate cells, located between the basal lamina and the endothelium II. Like pericytes in other parts of the body III. Especially common between two capillaries where they form a sheath shared by both capillaries IV. Contractile - Involved in regulation of GFR, Secretory, Take up immune complexes |
|
|
General
a. Juxtaglomerular apparatus - Components b. Lengths - PCT, DCT, Collecting duct c. Collecting ducts - Cells and their characteristics |
a. Juxtaglomerular apparatus
1. Macula densa of distal convoluted tubule 2. Lacis cells 3. Juxtaglomerular cells - Secrete renin, part of afferent arteriole b. Lengths I. PCT - 15 mm II. DCT - 5 mm III. Collecting duct - 20 mm long, pass through renal cortex and medulla to empty in renal pelvis c. Collecting ducts - Cells and their characteristics 1. Principal (P) cells I. Most numerous II. Tall, few organelles III. Involved in Na-reabsorption and vasopressin-mediated water reabsorption 2. Intercalated (I) cells I. Also found in distal tubule II. Microvilli, mitochondria III. Concerned with acid secretion and bicarbonate transport (Ganong) |
|
|
Renal circulation
a. Blood flow b. Glomerular capillary pressure - % of mean systemic pressure c. Regulation of the renal blood flow |
a. Just under 25%
b. 40% c. Regulation of the renal blood flow 1. NE I. Vasoconstrict, greatest effect on interlobular arteries and the afferent arterioles 2. Dopamine I. Made in kidneys II. -> Renal vasodilation and natriuresis 3. ATII I. Greater constrictor effect on the efferent arterioles than on the afferent 4. Prostaglandins I. Increase blood flow in the renal cortex and decrease blood flow int he renal cortex (Ganong) |
|
|
Dopamine - The 3 dosage regimens and their effect
|
Dopamine
1. Renal dose I. 2-5 ug\kg\min II. Primarily vasodilator effect on renal, mesenteric, and coronary vessels, natriuretic effect on kidneys III. Via D1 receptors 2. Cardiac dose I. 5-10 ug\kg\min II. Primarily +Inotropic and chronotropic effect on heart, via beta-1 adrenergic receptor III. Used for shock or heart failure 3. Pressor dose I. Primarily cause vasoconstriction, increased systemic vascular resistance, and increased blood pressure II. Through alpha-1 adrenergic receptors III. cause vessels in the kidney to constrict to the point where they will become non-functional (Wikipedia) |

