![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
3 Classes of membrane lipids
|
phospholipids (70-75%)
cholesterol (20-25%) glycolipids (2-3%) |
|
|
subclasses of phospholipids
|
choline-phospholipids: phosphatidyl-choline, sphingomyelins
amino-pholipids: phosphatidylserine |
|
|
function of cholesterol and saturated FA chains
|
adds stiffness, decrease membrane fluidity
|
|
|
where's glycolipids located
|
outer leaflet
|
|
|
where are phosphatidylserine and phosphatidylinositol
|
inner leaflet
|
|
|
where's sphingomyelin
|
external, some internal leaflet
|
|
|
the composition of membrane rafts and it's function
|
clusters of cholesterol, proteins sphingolipids
rafts provide island to cluster key signaling components, internalization with caveolae |
|
|
functions of membrane proteins
|
transport (pumps/carrier/channels)
structural - anchors, maintain membrane integrity receptors/signaling enzymes glycoproteins - Ab recognition |
|
|
types of membrane transport
|
diffusion
facilitated diffusion - channel, carrier active transport - primary, secondary |
|
|
random thermal Brownian motion
|
allow diffusion of molecules
|
|
|
Fick's First Law of Diffusion
|
J = -DA(dC/dX)
J - net rate of diffusion on moles or grams/time; D - diffusion coefficient of the solute A - area of the membrane dC - concentration difference across the membrane dX - membrane thickness |
|
|
D - diffusion coefficient of the solute, is inversely proportional to size and charge of particle and viscosity of solvent? (1/D)
|
T
|
|
|
permeability across membrane depend on ... (4)
|
size, charge, lipid solubility, membrane thickness
|
|
|
Facilitated diffusion, carrier proteins, transport solute via
|
ping-pong conformational change
|
|
|
Can facilitated diffusion and active transport reach Vmax?
|
Yes, b/c they are similar to enzyme
|
|
|
Facilitated diffusion and active transport exhibits 3 properties.
|
chemical specificity, stereospecificity, competitive inhibition
|
|
|
In simple diffusion, the greater the concentration difference, the ____ the rate of diffusion.
|
faster the rate of diffusion
|
|
|
the ion channel can be triggered open by .... (3)
|
voltage, ligands, stretch
|
|
|
1. primary active transport uses ___ as energy
2. secondary active transport couples |
1. ATP
2. energy from gradients |
|
|
examples of primary active transport, and its role
|
Na-K ATPase, moves 3Na+ out and 2K+ in. set up gradient across the membrane.
v-HATPase. moves H+ across gradient. |
|
|
Steps of Na+ and K+ transport by Na-K ATPase
|
1.Binding of 3 Na+ inside
2.Hydrolysis of ATP for energy 3.Release of Na+ outside 4.Binding of 2 K+ outside 5.Dephosphorylation 6.Release of K+ inside |
|
|
what chemical blocks Na-K ATPase? Which part of the pump does the chemical exhibit its effect? What is the effect on the heart?
|
oubain - digitalis glycosides
oubain binds extracellular side It slows heart rate - treat atrial fibrillation. |
|
|
v-HATPase function on bone and stomach.
What will happen if vHATPase are blocked with bafilomycin? |
It enhances bone resorption, and reduce acid release in the the stomach.
Inhibition will block tooth eruption |
|
|
Subsets of secondary active transport.
|
symport and antiport
|
|
|
example of symporter. how many Na+ are translocated per cycle.
|
Na/aa symport. 2 Na+ are translocated.
|
|
|
example of antiporter, and its functions
|
Na+/H+ antiporter, drives proton out of the cell.
|
|
|
Steps of transport of glucose from gut lumen into extracellular fluid. (active transport and facilitated diffusion)
|
Intestinal epithelium: [glucose] higher in than out
1. Na+ gradient established using primary active transport – ATP hydrolysis powers Na/K pump 2. Use energy of Na+ to get glucose into the cell using secondary active transport 3. Let glucose flow out down the gradient through carrier using facilitated diffusion |
|
|
Osmotic pressure
|
pressure needed to stop water movement.
|
|
|
osmolarity goes down refer to ...
|
in hypotonic solution
|
|
|
Osmotic pressure equation
|
p = RTfic
p - osmotic pressure RT - 22.4 atm f - osmotic coefficient i - number of ions formed by dissociation of solute c - molar concentration of solute |
|
|
Ions have both ____ and ____ gradients that must be balanced at equilibrium
|
electrical, chemical
|
|
|
chemical energy equation
electrical energy equation |
Chem energy F = -RT ln([G]i/[G]o)
Electrical energy F = zFV z = charge F = Faraday constant V = voltage |
|
|
Nernst equation
|

|
|
|
Ek in Nernst eqation is
|
Vm when major ions are at equilibrium, the potential at which there's no net movement of ions for the given intracellular and extracellular concentrations
|
|
|
typical cells' membrane potential at rest is at what range?
|
-50mV ~ -80mV
|
|
|
Which ions contribute to the membrane potential?
|
Na+, Cl-, K+, Mg++, Ca++
|
|
|
the more permeable the ion X, the ___ it contributes to the membrane potential
|
the more
|
|
|
in resting cell, ___ has the biggest influence on membrane potential, since it has the largest P.
|
K+ (or Cl-)
|
|
|
In resting cell, Vm is close to
|
Ek (equilibrium potential for K+)
|
|
|
when Vm = -60mV, resting membrane potential, are ions at equilibrium?
|
no, energy are required to pump ions against their concentration gradient
|
|
|
Goldman-Hodgkin-Katz (GHK) equation
|
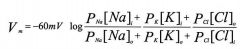
P = permeability coefficient
|
|
|
what sets resting membrane potential?
|
negatively charged proteins - impermeable
Na+-K+ pump - maintains gradients large K+ conductance (leak K+ currents add to negative membrane potential) - Vm near Ek |
|
|
impermeable intracellular anionic proteins will have what kind of effect of ions and water
|
cations will be attracted inside of the cell to balance out the charge, will allow water to move in by osmosis.
|
|
|
a small change in ion concentration makes a big change in membrane voltage. T/F
|
T
|
|
|
voltage ion channel structure
|
each domain has 6 segments span the membrane.
4 subunits (domains) create a pore |
|
|
key characteristics of ion channels ... (4)
|
gating, permeation/selectivity, inactivation, block
|
|
|
Gating of ion channels are mediated by (3)
|
secondary messenger (Ca2+), neurotransmitter, voltage change
|
|
|
how do ion channel discriminate against ions (permeation/selectivity)?
|
size of the ions, charge of the ion, and molecular interaction along the wall of the channel.
selectivity filter (close to extracellular space) bind to their ions. |
|
|
conductance of ion channels are
|
how quickly ions moving through the channel.
|
|
|
the more rapidly the ion move through the ion channel, ___ the conductance and ___ IpA
|
higher and higher
|
|
|
2 ways to increase total current across membrane
|
increase number of channels open
increase the amount of time each channel is open |
|
|
How is ion channels gated?
|
Ball and chain model of inactivation: positive protein loop moves into region exposed when voltage gates channel open.
|
|
|
tetradotoxin (TTX) blocks ___ channels
|
Na+ channel blocker
|
|
|
function of lidocaine derivative (QX-222) on ion channels
|
channel closes most of the time
|
|
|
effect of local anesthetics on ion channels
|
block movemnent of ions through pores
|
|
|
Ena
Ek Ecl Eca |
= + 60 mV
= – 86 mV = – 68 mV = + 125 mV |

