![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
181 Cards in this Set
- Front
- Back
|
SLOW WAVES:
- Are spontaneous rhythmical undulations of resting membrane potential around baseline of ______, generated by ___? |
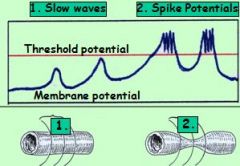
* undulations of resting membrane potential around baseline of (-50-60 mV)
generated by specialized smooth muscle cells (Cajal). |
|
|
How are slow waves conducted along a GI section (between cells)?
Frequency with which waves are generated? |
B/o Gap Junctions, slow waves are conducted along a GI section.
- Slow waves occur with a frequency of 3–12 / min depending on the gut section. |
|
|
critical threshold potential ?
what happens next? |
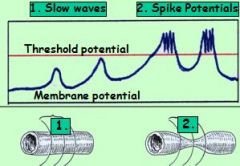
slow wave peak reaches critical threshold potential at (-40/–35mV) !!!!
* causes V/G Ca+ channels to open -> rapid influx of Ca+ causes spike potentials & muscle contractions, spread along GI section |
|
|
amplitude of slow waves can be modulated by
|
1. PRIMING FACTORS
2. Hyperpolarizing factors |
|
|
PRIMING FACTORS
|
PRIMING FACTORS which depolarize smooth muscle membrane -> more slow waves reach threshold -> GI motility increases: e.g. gut wall stretching, PS stimulation, some GI hormones
|
|
|
Hyperpolarizing factors
|
Hyperpolarizing factors -> harder for slow waves to reach threshold potention -> GI motility decreases, e.g. sympathetic stimulation, some GI hormones
|
|
|
GI motility involves two basic pattern:
|
1. Segmentation / Mixing (above): Localized contraction of mostly circular muscles
2. Peristalsis / Propulsion Circular contraction behind bolus; contraction travels toward anus over longer distance = strong local reflex called ADAPTIVE RELAXATION |
|
|
What can cause TRP (traumatic reticuloperitonitis)?
What results from this? |
if cow injests nail or foreign object, and it perforates reticulum
allows leakage of ingesta and bacteria, which contaminates the peritoneal cavity. inflammation can obstruct vagal innervation -> Receptor damage or inhibition -> motlility in reticulum decreases -> ruminal stasis (vagal indigestion) |
|
|
cell to cell signaling
system responsible to sending hormones via blood stream |
Paracrine
endocrine |
|
|
Most important DUODENAL factors for reducing stomach emptying rate are:
(not looking what is secreted, but for the specific things they stimulate e.g. receptor) |
All following are part of enterogastric inhibition:
- incr. pressure in duodenal lumen - low pH - HIGH FAT CONTENT IN DUODENUM - high peptide conc - high osmoregularity |
|
|
What happens if pH in gut (duodenum) too low?
|
: duodenal endocrine cells react to low pH values -> release Secretin into circulation -> stimulates pancreas and liver to release buffers into the gut
|
|
|
main excitatory neutrotransmitter of GI?
inhibitory neutrotransmitter of GI? |
(for most part actions of neurotransmitters in gut are reversed)
* Acetylcholine (mostly excitatory) * norepinephrine (mostly inhibitory) |
|
|
Myenteric plexus has what kind of receptors?
Efferent (motor) neuron are associated with what types of cells? What is influenced here? |
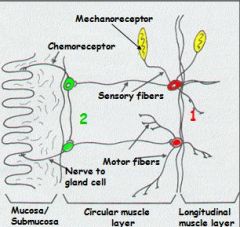
Mechanoreceptors monitor degree of muscle stretching -> Efferent (motor) neurons to muscle cells -> influence GI motility
Hint: Myenteric MMM |
|
|
Submucosal plexus has what kind of receptors? Efferent (motor) neuron are associated with what types of cells?
What is influenced here? |
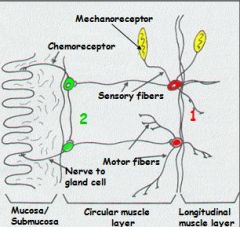
Chemoreceptors monitor chemicals (=nutrients, pH) -> Efferent (motor) neuron to secretory, endocrine and mucus cells -> influences GI secretions
|
|
|
stimulation of the parasympathetic nerves
|
mostly stimulates the GI’s functions, e.g.:
- increases motility - increases secretion rates - decreases sphincter tones |
|
|
Sympathetic fibers originate from ?
|
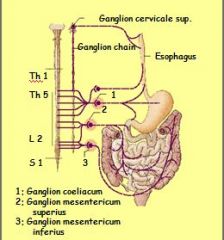
sympath. fiber originate between segments T5 to L2, and pass to mesenteric ganglia
|
|
|
stimulation of the sympathetic system causes what changes in GI function? sphincter tone? secretions?
|
stimulation of the sympathetic system
mostly inhibits activity of GI functions, e.g. - decreases motility - decreases secretions - increases sphincter tone |
|
|
Where are Crypts of
Lieberkuehn located? Name Cell types found there? (five of them) |
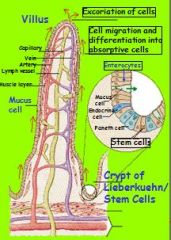
small intestine and colon (both)
STEM CELLS, found w/i the Crypts of Lieberkuehn differentiate into: 1. young enterocytes: secretory 2. mature enterocytes: absorptive; do not express BBM enzymes and transporters. 3. ENDOCRINE CELLS: produce endocrines, e.g. CCK, secretin 4. MUCUS CELLS 5. PANETH CELLS |
|
|
local reflexes controlled by
central reflexes controlled by ? |
Enteric NS
Autonomic NS - both use same receptors within the gastrointestinal wall , for most part |
|
|
Distension of the GI wall causes what chain of events ?
(looking for specifc factor) |
stimulation via mechanoreceptors -> release of priming factors -> opening of ligand-gated ion channels -> depolariz. of smooth muscles membranes -> passing slow waves will reach threshold -> spike potentials -> contractions
|
|
|
inflammatory mediators of GI?
|
prostaglandins
histamine cytokines |
|
|
inflam. mediators act as _____crines, typically stimulating secretory and motility functions of GI, leading to ______
|
inflam. mediators act as PARACRINES (cell to cell signaling) typically stimulating secretory and motility functions of GI, leading to PERISTALTIC RUSH (diarrhea)
|
|
|
PERISTALTIC RUSH
|
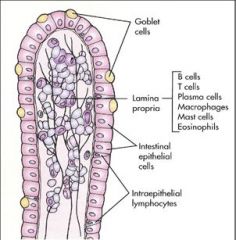
powerful and rapid peristaltic movements of sm. int. caused by intense mucosal inflammation = protective response to quickly push potentially harmful contents into Large Intestines
|
|
|
How could Large Redworm (Strongylus vulgaris) have lead to an infarction within the large colon??
note: Large Redworms larvae live within a horse’s vascular system at the aorta / intestinal artery junction. |
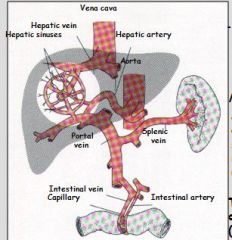
Need to burrow through endothelium of artery to get to mucosa -> tissue damage & clot formation -> blockage of GI arteries -> lack of perfusion -) ischemia (lack of blood supply)
|
|
|
How can hypoxia of tissues (affected by redworm) lead to necrosis?
|
hypoxia=> anaerobic metabolism -> lack of ATP and increase in lactic acid -> inhibition of na/k pumps and other enzymes/proteins with intracellular accumulation of na -. Cellular swelling and death.
|
|
|
Septicemia
|
presence of pathogenic organisms in bloodstream, leading to sepsis
|
|
|
How does saliva of animals differ from humans?
(give specific pH) Which ions are secreted? |
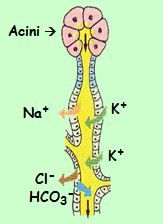
Animal Saliva is slightly hypotonic, slightly alkaline
(pH 7.2-8.4), and K-rich Acini secrete mucus & K+ and HCO3- , while Na+ & Cl-taking up |
|
|
diagnostic indicator of liver disease
|
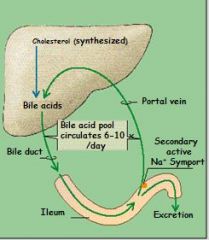
increased amount of bile acids in plasma
-> b/c damaged hepatocytes cannot extract bile acids from portal blood |
|
|
“warming up” phase (of digestive organs) innervated by what cranial nerve? ...duh
|
CN X and initiate VAGAL REFLEXES
leading to mild stimulation of the stomach, small intestines, pancreas and bile = “warming up” phase |
|
|
Why do ruminants produce continuously large volumes of alkaline saliva rich in bicarbonate & phosphate buffers?
|
essential to neutralize
fermentation products in the rumen! (i guess products cause low pH) |
|
|
Give 3 or 4 examples of what might cause megaesophagus ?
|
e.g. caused by:
- Achalasia: lower sphincter fails to relax due to genetic malfunction of myenteric plexus - or a Persistent Right Aortic Arch: mechanical obstruction - myasthenia gravis (acetylcholine receptor deficit or damage) neuromusclar disorder, means "grave muscle weakness", problem with vol.musc. - idiopathic |
|
|
Which clinical signs would lead to suspicion of a PRAA?
|
regurgitation after eating malnutrition (coughing, aspiration of food, pneumonia)
|
|
|
At which age would PRAA disorder typically show up?
|
at time of weaning.
(b/c of eating solid food) |
|
|
Describe a cross section of a typical gut wall
|
Consists of an outer layer of longitudinal muscle, and inner layer of circular muscle and a lamina muscularis mucosa
|
|
|
Lipids (triglycerides) broken down into
|
Lipids (triglycerides) broken down into monoglycerides and fatty acids (2)
|
|
|
-slow waves are ?
(how many / minutes?) |
-slow waves are spontaneous rhythmical undulations of the resting membrane potential, generated by specialized smooth muscles (3-12/min)
|
|
|
-spike potential is reached and a contraction happens only when a ?
|
when a slow wave peak reaches critical threshold potential (-40/-35mV), voltage gated calcium channels open -> rapid influx of calcium causes spike potentials and elicits muscle contractions
|
|
|
Priming factors include:
* leads to ? |
Priming factors include: any stimulation (of mechano- or chemoreceptors) that bring the slow waves closer to threshold!
e.g. gut wall stretching, parasympathetic stimulation, some GI hormones * leads to increased motility |
|
|
salivary amylase (omnivores only)
|
salivary amylase (in omnivores only) start carbohydrate digestion!!
|
|
|
purpose of esophageal sphincters?
|
* Upper and lower esophageal sphincters are tightly closed when not in swallowing process
* avoid entry of air and esophageal reflux of gastric contents |
|
|
Name the digestive functions of the stomach
|
1. Temporary storage of food
2. Mixing of food with gastric secretions and liquefaction of ingesta into chyme 3. Chemical and enzymatic digestion, particularly of proteins 4. Controlled release of liquefied chyme into the small intestines |
|
|
IS absorption a physiological gastric function?
|
NO!
|
|
|
3 functions of gastric motility
|
1. Mixing (distal stomach) = food stim. enhancement of peristalsis originating in pacemaker area
2. Release of chyme: mixing slowly liquefies content 3. During fasting phases (empty stomach): occas. strong peristaltic waves occur btw. stomach and ileum while pylorus is relaxed= MMC |
|
|
Parietal cells secrete?
|
HCl and Intrinsic factor
|
|
|
Enterochromaffin-like cells secrete?
|
Enterochromaffin-like cells: Histamine
|
|
|
Chief cells secrete
|
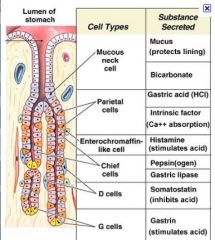
Chief cells: Pepsinogen or Prochymosin
|
|
|
G (endocrine) cells secrete:
why? from where? |
G (endocrine) cells: Gastrin, pyloric region only
- released in resp. to high polypeptide content |
|
|
principle functions of Histamine
released in response to? |
released from enterchromaffin cells (strong stimulus), released in response to Gastrin
histamine is inflammatory mediator, act as paracrines, typically stimulating secretory (HCl!!!) and motility functions of the GI, leading to : |
|
|
Principle functions of Gastrin:
What stimulates its release? What is released is response to gastrin secretion? What inhibits Gastrin release? |
Released from G cells into blood in response to peptides (mild direct stimulus). increases HCl secretion and gastric motility.
Histamine is released from EC cells in response to gastrin. When pH < 2, Gastrin release becomes blocked and vagal and local reflexes become inhibited to prevent excessive acidification. (Self-limitation of stomach) |
|
|
3 control phases of gastric functions
|
1. Cephalic phase: anticipation of food, etc.
2. Gastric phase 3. Intestinal phase=when chyme arrives in duodenum |
|
|
Describe functions of Gastric phase:
|
arrival of food in stomach, stimulation of gastric mechano and chemoreceptors leading to strong stimulation of local (plexus) and central (vagal) reflexes.
Effects: relaxation of muscles (accommodation) in proximal parts Vigorous muscle contractions (mixing/grinding) in distal parts String stimulation of all gastric secretions with massive HCl release |
|
|
Describe enterogastric reflex:
What is transmitter? Where is receptor located? Effector? |
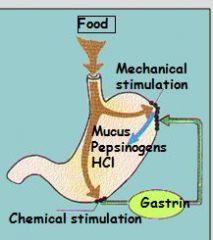
Inhibitory stimulus from SI to stomach; transmitted by secretin and cholecystokinin (CCK) to reduce gastric secretions.
|
|
|
Storage occurs mostly in proximal stomach,
facilitated by the ______. |
Accommodation reflex
reduction in muscle tone in proximal parts -> relaxation -> expansion of stomach w/o pressure increase |
|
|
Describe functions of Intestinal phase
|
Intestinal phase=when chyme arrives in duodenum -> inhibition of gastric glands and motility due to activation of receptors in the small intestines=prevents overloading of small intestines (negative feedback)
Stimuli: Distention of small intestines High acidity High nutrient content Irritation of mucosa =enterogastric reflex |
|
|
G (endocrine) cells?
|
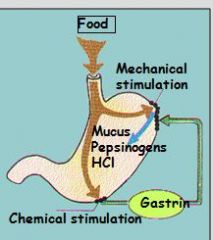
Gastrin
Gastrin released from G cells into blood in response to peptides (mild direct stimulus) Gastrin increases HCl secretion and gastric motility |
|
|
Explain the functions of hydrochloric acid
|
- To macerate ingesta into fluid
- To denature proteins (quatrenary and tertiary structure) - To begin emulsification of fats - To activate the proteolytic enzymes pepsinogen or prochymosin - To be bacteriacidal |
|
|
principle functions of Intrinsic factor
|
released from parietal cells, co-secreted with HCl, essential for absortption of vit B-12 -> necessary cofactor for erythropoiesis
|
|
|
principle functions of prochymosin
|
nursing animals secrete procymosin instead of pepsinogens (rennin in calves), specific for coagulation of casein
|
|
|
ENTEROCHROMAFFIN-LIKE cells secrete? (In response to what?)
What are affects? |
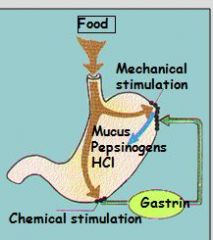
Histamine
Histamine is released in response to Gastrin (= strong stimulus); like gastrin promotes HCl secretion but in as paracrine (cell to cell) "effect of gastrin is partly - or entirely - mediated by histamine" - Fig.14.35, p.535 |
|
|
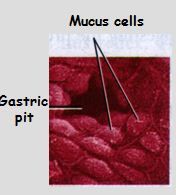
SECRETION OF MUCUS is controlled by
(name cells and specific hormone promoting secr.) |
SECRETION OF MUCUS is controlled by local reflexes (enteric NS)
and central reflexes (Vagus): lead to synthesis of prostaglandin (PGE2) in goblet cells -> increases mucus & bicarbonate release. |
|
|
goblet cells produce ?
|
MUCUS
synthesis of prostaglandin (PGE2) |
|
|
Parietal cells secrete what into stomach lumen?
|
HCl + Intrinsic factors
|
|
|
How does the local and central reflexes control motility (increase it)?
|
It leads to synthesis of PROSTAGLANDIN (PGE2) in goblet cells which increases mucus and HCO3- release
|
|
|
Which drugs interfere with PG synthesis (and thus release of mucus and HCO3-)?
|
NSAIDS
|
|
|
principle functions of pepsinogens
|
Pepsinogens: prescurors of pepsins
* activated in low pH * are proteolytic enzymes -> initiate protein digestion (hydrolysis of collagen by splitting large ppt. into smaller ppt. and some oligopeptides |
|
|
which part of stomach is non-glandular?
|
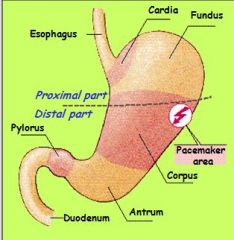
distal
|
|
|
Semi-solid and solid foods require
grinding and mixing with gastric juices into fluid ____ before it can pass the pylorus (= strong, sieve-like sphincter) |
mixing with gastric juices into
fluid CHYME before it can pass the pylorus |
|
|
Migrating motor complexes
|
Occasional strong peristaltic waves occur between stomach and ileum while pylorus is relaxed
> clear stomach and small intestine of undigested food particles |
|
|
interstitial Cajal cells
|
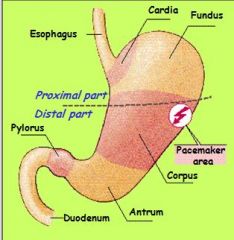
PACEMAKER CELLS that lie between circular and longitudinal muscle layers in both stomach and intestines.
conduct repetitive and spontaneous oscillations in membrane potential transmitted thr. gap junctions. Ca+ flowing into muscle during AP will induce CONTRACTION |
|
|
Name a few (of 35) regulatory peptides of GI:
|
Gastrin, Somatostatin, Secretin,
Cholecystokinin, Gastric inhibitory peptide, Motilin, Enteroglucagon, Peptide YY etc |
|
|
Which have positive effect on HCL secretion?
A. histamine B. Gastrin C. NSAIDs D. sympathetic innervation E. acetylcholine |
histamine, gastrin, acetylcholine
- all req. for MAX secretion of HCl |
|
|
location of "vomiting center"
|
brain stem
|
|
|
CONSEQUENCES OF SEVERE VOMITING
|
loss of fluid -> hypovolemia -> blood pressure down / circulatory shock
loss of K+ -> hypokalemia -> hyperpolarization of membranes -> reduced excitability of nerve/muscle cells -> weakness + hyporeflexia loss of H+ -> metabolic alkalosis -> hypoventilation |
|
|
COMPONENTS of Gastro-Intestinal barrier are:
|
- Mucus and Bicarbonate –
- Prostaglandin E2: incr. blood flow, mucus and HCO3 secretions - Epithelial cells w/tight junctions and stem cells = high regen. capacity - Bactericidal peptides / immunoglobulins A |
|
|
causes of disruption to barrier
therapy? |
NSAIDs (inhibit PGE2 which promotes mucus/HC03), stress, ISCHEMIA, INFECTIONS
THERAPY: stress relief, histamine (H2) blockers (histamine, released b/c of irritation, promotes HCl secretion), proton pump blockers, antibiotics (if infection is cause of probkem) |
|
|
Purpose of Co-operation between the PANCREAS, BILE and small intestinal MUCOSA?
|
1. Neutralization of gastric acid (via buffers) to avoid mucosal damage
2. Hydrolysis of macromolecules into micromolecules for absorption |
|
|
pancreatic enzymes to function, which require pH of?
this is enabled by ? |
pH > 5
BICARBONATE Solution: secreted by the pancreatic duct system to (neutralizes gastric acid) |
|
|
3 major groups of DIGESTIVE ENZYMES produced in the pancreatic Acini are ?
|
The 3 major groups of DIGESTIVE ENZYMES are amylolytic, proteolytic and lipolytic
|
|
|
a-Amylase digests ?
Where is secreted from? pH requirement? |
* a-Amylase digests most soluble but not structural carbohydrates
* Amylase splits polysaccharides into oligosaccharides * secreted from pancreatic duct system, req. pH >5 |
|
|
name some PROTEOLYTIC ENZYMES
|
Trypsinogen, Chymotrypsinogen, Pro-Elastase, Pro- Carboxypeptidases, etc
|
|
|
Proteolytic enzymes are secreted in vesicles as inactive pro-enzymes for what reason?
Proteolytic enzymes produce mostly? |
to avoid autodigestion
Proteolytic enzymes produce mostly oligopeptides |
|
|
stimulation of the exocrine pancreas begins with ?
MAIN PHASE of pancreatic stimulation ? via what 2 hormones? |
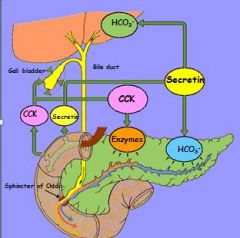
CEPHALIC PHASE (“warming up”).
INTESTINAL PHASE = MAIN PHASE of pancreatic stimulation via 2 hormones: SECRETIN and CCK |
|
|
Direct digestive functions of the LIVER are:
|
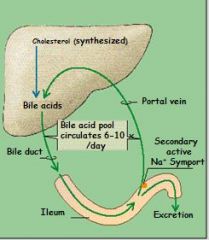
- to facilitate digestion and abs. of fats and lipophilic vitamins via SECRETION of BILE ACIDS
- to neutralize acidic chyme via bicarbonate secretion |
|
|
What cells secrete bile juice (including bile acids and other components)? What does bile juice consist of?
Not asking structure of bile acids...that's a seperate question. |
1. Hepatocytes secrete
- Bile acids (> 50% of solutes) - Lipophilic waste products, e.g bile pigments = bilirubin, cholesterol etc 2. Duct cells secrete - Bicarbonate - Electrolytes and water |
|
|
BILE ACIDS are derived from
|
cholesterol
|
|
|
cholesterol conjugated with A.A.to form
|
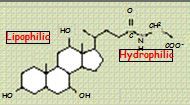
BILE ACIDS!! -> DETERGENT = has hydrophilic and lipophilic end
|
|
|
Bile acid RECYCLING:
bile acids are re-absorbed in ___, re-enter _____and are secreted again and again (up to 20 times). |
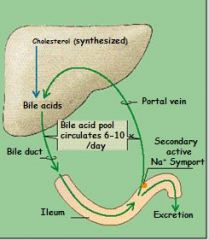
95% of bile acids are re-absorbed in ILEUM, re-enter LIVER and are secreted again and again (up to 20 times).
|
|
|
The re-uptake of bile acids into liver stimulates _____ to secrete more bile juice.
|
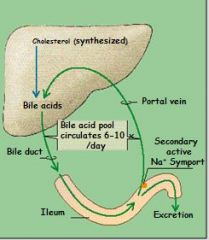
The re-uptake of bile acids into liver stimulates HEPATOCYTES to secrete more bile juice = positive feedback
|
|
|
salivation controlled by what cranial nerves?
|
When chewing and/or salivating parasympathetic efferents via
CN VII & IX -> more salivation |
|
|
STEM CELLS differentiate into ?
(just names and type of cell) |
o Young enterocytes -> Secretory cells!
(line Crypts of Lieberkuehn) o Mature enterocytes -> Absorptive cells -> BBM |
|
|
Stem cells also differentiate into:
|
ENDOCRINE CELLS: produce endocrines, e.g. CCK, secretin
MUCUS CELLS PANETH CELLS |
|
|
YOUNG ENTEROCYTES secrete ?
|
* secrete large volumes of INTESTINAL FLUID to facilitate digestion & abs. through active Cl secretion into gut lumen -> creates electrical gradient -> pulls Na+ in -> H2O follows (ca. 2 liters/day/man) -> dilution of chyme!!
Note: some toxins, inflam. mediators and viruses stimulate Cl- transport |
|
|
What are only enzymes that require very low pH (1-3)?
|
pepsinogens
|
|
|
What does cephalic phase entail?
|
- chewing, swallowing
- mechan. and chem. (in some sp.) digest. begins - "warming up" during which signals sent stomach, acc.glands, even S.I. to prepare for food! |
|
|
Why is myasthenia gravis a problem in pregastric digestion?
Breed most common in? |
AcH receptor deficit, so problem with motor end plate, also problem getting into stomach
- seen in sharpe' most |
|
|
What are secretions that also occur during cephalic phase?
|
mucus, gastrin, histamine, HCl, pepsinogen
- secretions start (mildly) after stimulation of vagus nerve by mechano- and chemoreceptors - although this secretions most likely intensify during gastric phase (when food how actually enter stomach) |
|
|
What is unusual about vagus nerve stimulation and accomodation reflex?
|
- in digest., vagal innervation usually leads to smooth muscle constriction
- during acc.reflex, see red. in musc.tone (relax) - likely uses different nt's e.g. nitric oxide or VIP |
|
|
1. Where does salivary amylase work?
2. What exactly does it break down? Bonds? |
up to proximal stomach during storage phase where pH not too low ~30% digested
(expand on this) a-Amylase hydrolyses only a-1-4 linkages of polysaccherides; products are oligo- and some disaccharides (mostly maltose), which cannot be absorbed |
|
|
How does emulsification work?
Objective here? |
* Emuls. referes to process of mixing two or more immiscible (unblendable) liquids
* Bile acids attach to surfaces of fat globules -> vigorous mixing in aqueous chyme -> globules break up into smaller pieces -> increases S.A. for action of pancreatic lipases! * after hydrolysis by lipases, form micelles and can be transported in aqueous layer with chyme |
|
|
MATURE ENTEROCYTES secrete? what is their purpose?
|
- develop microvilli -> BBM
- containing digestive enzymes and absorptive mechanisms. - products of LUMINAL digestion hydrolyzed further, to be absorbed |
|
|
trypsinogen is secreted by?
activated by? what stimulates it release? what trypsin do? |
trypsinogen is secreted by pancrease in vesicle with inhibitory compound; activated into trypsin by enteropeptidase (from SI.).
- trypsin formed first stim. further release of trypsinogen and rest of proteolytic enz. |
|
|
what is luminal phase of digestion?
|
Luminal phase = macromolecules e.g. polysaccherides into smaller but still not absorbable pieces; e.g. oligo- and some disaccharides.
|
|
|
Brushborder phase of starch digestion ?
(what bonds broken here?) |
Oligo- and disaccharidases within the BBM (e.g. maltase) hydrolyse a-1-4
and a-1-6 linkages; endproducts are monosaccharides (Glc) = ABSORBABLE MONOMER |
|
|
Can dietary DISACCHARIDES (sucrose and lactose) be hydrolyzed by alpha-Amylase?
|
NO, require disaccharidASE in BBM.
|
|
|
Absorption of Glucose and Galactose into absorptive cells occurs
via ? |
secondary active Na-cotransport mechanism,
which depends on electrochemical SODIUM GRADIENT, created by the Na/K pump at the basolateral side. ~requires intact enterocytes |
|
|
Fructose is absorbed via ?
|
facilitated diffusion.
~requires intact enterocytes |
|
|
Newborns of some species can absorb colostral immunoglobulins (by endocytosis). The digestion of these proteins is avoided by:
|
- limited HCl secretion
- limited pro-chymosin secretion - a trypsin inhibitor in the colostrum |
|
|
how can toxin affect absorption?
|
Many of these transporters are inhibited by enterotoxins except for the
Na-Glc transporter ! |
|
|
Process of Fat digest and absorption:
|
1. Fat globules EMULSIFIED by HCl and conj. bile acids
2. Pancr.lipase / colipase attach to fat globule surface; HYDROLYSE triG into MONOGLYCERIDES & free FA 3. micelles 4. transport to BBM and diffuse in 5. Free conj. bile acids return to emulsify more fat globules or form new micelles |
|
|
after being absorbed into enterocytes:
Triglycerides, cholesterol, phospholipids, fat-soluble vitamins and newly formed lipoproteins aggregate into ? |
Triglycerides, cholesterol, phospholipids, fat-soluble vitamins and newly formed
lipoproteins aggregate into CHYLOMICRONS |
|
|
How is water reabsorbed in sm.int.?
|
secretions in proximal part greatly incr. osmoreg, so eventually electrocytes (mainly Na+) diffuse back into cells passively -> water follows
|
|
|
By the time chyme leaves
the small intestines, ca ____% of its water content have been reabsorbed! |
80-90% of water content
have been reabsorbed in sm. intestine! |
|
|
SECRETORY Diarrhea
|
Direct stimulation/irritation of secretory/mucus cells by enterotoxins (from e.coli), inflammatory mediators, etc to secrete more fluid
|
|
|
Why would SECRETORY Diarrhea cause metabolic acidosis?
what else happens? |
bicarbonate loss (leaves before can be reabsorbed) -> metabolic acidosis
fluid loss -> hypovolemia -> hypoxia/metab.acidosis/tissue damage + shock and circulatory collapse also K+ loss -> hypokalemia -> hyperpolarization of nerve/muscle membranes -> weakness, hyporeflexia |
|
|
OSMOTIC (malabsorptive) Diarrhea
Causes? |
gut lumen contains large # of molecules which are poorly
absorbed -> high osmolality of chyme -> water is “sucked” into lumen - accum. of partially digested ingesta due to e.g.pancreatic deficiency (GI mucosa is intact) - loss of absorptive cells due to mucosal damage (inflammations, infections/ some toxins) -> digestion cannot be completed and micromolecules cannot be absorbed |
|
|
GASTRO-ILEAL REFLEX
|
At end of SI digestion, animal will take in next meal -> gastric distension -> SPHINCTER OPENS and ingesta is propelled into caecum
|
|
|
serotonin, dopamine, CCK, ATP, somatostatin,bombesin, motilin, substance P, vasoactive intestinal peptide, etc are examples of ?
|
neurotransmitters
|
|
|
What is secreted by cells of LI to help motility and protect cells?
|
Bicarbonate-rich fluid and mucus: buffering of
fermentation products (VFA's!!!!) to protect mucosa |
|
|
Vitamin produced by bacteria of LI
|
Vit K
|
|
|
Absorption in LI regulated by:
|
Sodium via active Na-K pump, followed by chloride and water; pump is regulated by ALDERSTERONE
Aldersterone stimulates uptake of sodium; H2O follows Na+ |
|
|
How can psychogenic tension produce diarrhea?
|
parasympathetic stimulation -> increased HCO3 secretion
|
|
|
2 reflexes involved in defecation?
which is more significant or supports other? |
1. Myenteric reflex:
pressure receptors in rectum -> stimulates myenteric plexus -> peristalsis & internal sphincter relaxation -> defecation (myenteric reflex is a weak reflex, needs to be fortified by parasympathetic reflex) 2. PS reflex (via pelvic nerves): Rectum distention -> PS. fibers / pelvic nerves -> intensified peristalsis |
|
|
What origin of pelvic nerve?
|
S1-S3
|
|
|
Defecation reflex can be blocked by voluntary constriction of
|
external sphincter and puborectal muscles (via pudendal nerve)
|
|
|
Pelvic Nerve damage leads to?
|
Pelvic Nerve damage -> paralyses defecation reflex -> constipation (this is more dangerous than incontinence, but the latter leads to more euthasia)
also affects bladder, urine dribbling, urinary incontinence |
|
|
Pudendal Nerve damage
|
Pudendal Nerve damage -> abolishes control -> incontinence
~b/c this is nerve that innervates external sphincter |
|
|
Polydipsia
|
thirst
|
|
|
hepatitis
|
inflammation of liver char. by presence of inflammatory cells in tissue of organ.
Can be self-limiting or progress to fibrosis (scarring) and cirrhosis. |
|
|
Cholangitis
|
Cholangitis is an infection or inflammation of common bile duct
|
|
|
Cholestasis
|
cholestasis is condition where bile cannot flow from the liver to the duodenum.
2 types: 1. due to obstruction e.g. gallstone or malignancy 2. metabolic types: disturbances in bile formation b/c of genetic defects or acquired as side effect of many medications. |
|
|
Neoplasm
|
abnormal growth e.g. tumor
|
|
|
Polyphagia
Steatorrhea |
excessive hunger
excess fat in feces, often due to lack of bile acids due to any number of causes e.g. cholangitis, cirrhosis, |
|
|
What are 4 zones (of stratification) for ruminal ingesta?
|
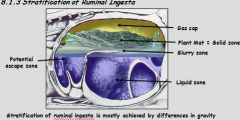
1. Gas cap: microorganisms produce gas during fermentation
2. Solid zone: dorsal rumen receives undigested plant matte 3. Liquid zone 4. Potential escape zone |
|
|
Potential escape zone:
What gets out? Where does it go? |
only sufficiently fermented (=small) particles and fluid moves from here into the omasum
|
|
|
In what breed is exocrine pancreas insufficiency common?
what condition is corollary of EPI? |
german shepards
diabetes mellitus (due to insulin loss) |
|
|
Fermentation products?
|
VFA's, CO2, CH4
|
|
|
VFA's are absorbed via ?
|
simple diffision
|
|
|
hindgut inflammation
can lead to? |
colitis e.g. caused by bacterial infections
diarrhea |
|
|
RUCTUS = ?
What does it refer to? |
belching? or removing of gas byproducts from fermentation
Secondary Contraction = B-cycle = Eructation contraction of the Reticulorumen and Ructus |
|
|
What happens during A-cycle of rumen contraction?
|
1. contraction of reticulum, dilation of omasal orifice
2. light, plant material (on top) pours into dorsal sac; heavy stuff remains 3. cranio-caudal contraction: squeezes rest into ventral sac to ferment 4. contraction of ventral sac pushes toward reticulum where cycle starts again 5. fluid and finer material flows into omasal orifice |
|
|
Where is omasal orifice located in cow stomach?
What passes through here? |
in Reticulum/Reticulo-ruminal fold, near cranial sac
only sufficiently fermented (=small) particles and fluid move from here into the omasum |
|
|
How many A-cycles are completed before can proceed to B-cycle?
|
2-3 A-cycles
|
|
|
During B-cycle (eructation), what allows sphincter relaxation and escape of gas into esophagus?
|
gas forward to the cardia
region -> activates cardia receptors -> sphincter relaxation -> gas enters esophagus -> antiperistaltic wave moves gas orally |
|
|
Why is bad idea to keep cow in lateral recumbency during anesthesia?
What can happen? |
Inhibition of ructus -> obstruction of esophagus, ruminal stasis) -> accumulation of gas -> BLOAT
-> pressure on thoracic organs -> circulatory failure |
|
|
what reflex controls the reticulorumen motility?
|
Vagal reflex
-controls the reticulorumen motility, involving - receptors in the mouth, forestomach, stomach, s.I. - vagal afferent (=sensory) fibers - motility centre in the medulla oblongata - vagal efferent (=motor) fibers |
|
|
pH range of reticulorumen?
what happens if outside this range? |
Chemo receptors: monitor pH (normal range 5.5-6.8);
depress motility when pH falls < 5.0 to slow down fermentation |
|
|
Is reticulorumen motility spontaneous or does it react to stimuli?
What drives it? |
Motility centre shows no spontaneous activity = needs to be driven by excitatory signals from receptors
|
|
|
what is strong local reflex
______ related to peristalsis? |
ADAPTIVE RELAXATION
relaxation caudal to bolus |
|
|
Clinical signs of vagal indigestion or traumatic reticuloperitonitis?
|
Motlility in r/r decreases so:
§ But the omasum is controlled by intrinsic ns and keeps contracting and taking in material and if reticulum hasn’t contracted,plant mater will enter omasal orifice § As omasum opens and sucks in plant matter: passes to aboomasum, □ Unfermented / udigested plant material in feces!!! |
|
|
max retention time of digesta in reticulorumen?
max retention time of digesta in omasum? |
72 hours, for really coarse fiber
(lush grass or concentrates) Retention time ca. ½-3 hrs of omasum |
|
|
Ruminal Bacteria types
|
ca. 200 species; mostly anaerobic , grouped
according to function, e.g.: cellulolytic, amylolytic, proteolytic, methanogenic, etc |
|
|
ADH causes what feeling ?
What else? What reflex do these stimulate? |
thirst
also coppersalts and sodiumsalts reticular groove reflex: groove blocks off rumen, so indigested material bypasses rumen and goes right to omasum (protects to oral drugs or milk in neonates) |
|
|
Maltose
|
disaccharide formed from two units of glucose joined with an α(1→4)bond.
|
|
|
lactose is ?
|
disaccharides (formed from galactose and glucose)
|
|
|
name 3 structural carbohydrates
|
Cellulose
Hemicellulose Pectin (structural Ch, = containing B- linkages) |
|
|
name 3 main VFA's and their relative %
|
BUTYRATE 10%
PROPRIONATE 20-30% ACETATE 60-70% |
|
|
If feed cow lots of grains, propionate and Acetate can form from ?
What happens if feed too much starch and grains? |
lactate, b/c low pH favors lactic acid producing bacteria
fermentation also occurs rapidly, more VFA's form faster than can be absorbed and buffered, H+(out)/Na+(in) exchanger overloaded, so pH drops, RUMEN ACIDOSIS, Drop in pH inhibits Na+/ K+ pump, leading to further accumulation of H+ and of Na+ , Intracellular Na , edema, => necrosis (ruminitis) |
|
|
fermentation yields ATP via the main ____ pathway
|
acetate
|
|
|
partly utilize ATP to regenerate which reduced co-factors ??
Where do co-factors come from? Why regenerate co-factors? |
(NADH2 -> NAD+)
via proprionate and butyrate to keep pathways open |
|
|
Main purpose of omasum?
Where are microbes digested? |
absorption of VFA's, bicarb, H2O
abomasum, via HCl |
|
|
what do ruminal microrobes require?
|
NH3 and VFA's (which they produce)
|
|
|
NPNs (urea, nitrates) supply
is achieved via: |
1. Dietary Supplementation
2. the Rumino-Hepatic Nitrogen re-cycling: |
|
|
If ruminal NH3 concentration is low ->
|
If ruminal NH3 concentration is low -> urea is secreted directly or via saliva into the rumen for bacterial growth.
|
|
|
If ruminal NH3 concentration is high ->
|
If ruminal NH3 concentration is high -> urea is excreted via kidneys.
|
|
|
Where do ruminants get protein?
|
digest microbes in SI, abomasum, omasum
|
|
|
enteric IMMUNE system release what inflammatory mediators?
|
if challenged, releases inflammatory mediators, e.g. prostaglandins, histamine, cytokines, etc
|
|
|
2 strong vasodilators of GI blood supply?
GI BLOOD FLOW is also stim. by? |
several endocrines, i.p. by bradykinin and kallidin
* decreased oxygen / increased CO2 conc. during activity phases * pumping action of GI movements |
|
|
2 common tumors in dogs
|
mast cell tumor and gastronoma
|
|
|
What % of VFA's are absorbed in r/r?
in omasum? |
70-85%
8-18% |
|
|
in forestomach (r/r) which form of VFAs absorb better (ionized or undissociated)?
|
undissociated
|
|
|
Because pka value for VFA (4.6-4.8) far below normal rumen pH, the proportion of undissociated acid _____ when pH falls after feeding (esp. if a lot of grains).
What does this mean in terms of absorption? |
increases when pH falls, b/c acids happy at low pH
undissociated = readily absorbed |
|
|
at pH 6.8 high roughage diet yield higher proportion of which VFA?
|
more acetate!!! (dissociated or ionized form of acetic acid)
- not abs. easily |
|
|
grass tetany
what is it? caused by? |
Low concentration of Mg+ or low Mg/Ca ratio in ECF leads to influx of Ca+ into nerve ending and increase AcH release. Tetany char. by involuntary contraction of muscles,
Why? Mg abs. reduced if feed contains high K+ (e.g. fertilizer), or if cow feeding on alot of immature grass. |
|
|
how does acetyl choline affect secretions in stomach? how does it stimulate?
|
AcH stimulates chief cells, parietal cells, and mucin-producing cells, AcH (like histamine) binds to receptors e.g. on parietal cells and evoke secretion.
|
|
|
rennin does what?
|
young ruminant produce rennin which in acidic environment cause precip. and coag. of milk protein casein. Coag. incr. retention in abomasum and allows for more complete digestion
(pepsin also coagulates milk but action is much slower) |
|
|
endocrine portion of pancrease produces?
exocrine portion produces? |
insulin and glucagon
pancreatic juice, which has important digestive functions |
|
|
where is bile made?
|
liver, but stored in gall bladder
Although bile duct may join pancreatic duct (which secretes pancr.juice) in some species, don't confuse them. Bile does NOT come from pancreas. |
|
|
where is CCK secreted from?
ALL functions of CCK? ALL targets? |
Secreted by endocrine cells of proximal small intestine (found in Cr. of Lieberkuehn)
1. When chyme in duodenum, releases CCK, causes gallbladder to release conc. bile. 2. causes incr. release of pancreatic enzymes 3. reduces gastric motility |
|
|
When is secretin released?
From Where? all functions of secretin? targets? |
WHEN PH LOW SECRETIN RELEASED FROM DUOD. in blood:
1. main target for secretin is pancreas, to secrete HCO3-rich fluid, which flows into prox. intestine thr.pancreatic duct. acts as buffer. 2. stimulates LIVER to release buffer 3. reduces gastric secretions |
|
|
trypsinogen comes from?
When is required to activate trypsinogen? |
trypsinogen from pancreas
requires enteropeptidase from intestine to make active trypsin |
|
|
Where are bile salts reabsorbed. (to be recycled)?
Why bile salts and not bile acid form? |
bile salts reabs. and returned to liver by portal vein
Bile salt is ionized form, not soluble in fat, so remain in small intestine (not reabsorbed) until reaches distal end |

