![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Approximately how much salt is in 70 kg human body?
|
4,9 kg
|
|
|
Approximately how much protein is in 70 kg human body?
|
12,6 kg
|
|
|
Approximately how much water is in 70 kg human body? And how much it is in percentage?
|
42 L, 60%.
|
|
|
Approximately how much fat is in 70 kg human body?
|
10,5 kg
|
|
|
Name at least 6 properties of cell.
|
Growth;
Assimilation of materials from the environment; Excretion waste products; Reproduction; Response to changes in the environment; May exhibit motility. |
|
|
Water is polarised, what does it mean in connection with ions?
|
It is suitable for dissolving ions.
|
|
|
Express body fluid compartments in percentage.
|
60% of TBW (total body weight) is water;
from it 40% is ICF and 20% is ECF. So there is more water inside the cells that outside. |
|
|
What parts does ECF consist of?
|
Plasma (within blood vessels); interstitial fluid (surrounding cells); transcellular fluid (e.g. synovial fluid, joints, brain, heart).
|
|
|
What separates ICF from ECF?
|
Cell membrane.
|
|
|
There is more ... in ICF than in ECF.
|
Potassium, K.
|
|
|
Try to draw a system of homeostatic mechanisms.
|
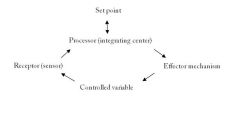
|
|
|
Name the composition of the ECF, of Na, Ca, K, HCO3, Cl in mEq/L.
|
Na = 140
Ca = 2,5 K = 4 HCO3 = 24 Cl = 105 |
|
|
Name the composition of ICF, of Na, Ca, K, HCO3, Cl in mEq/L.
|
Na = 14
K = 120 Ca = 0,0001 Cl =10 HCO3 = 10 |
|
|
What does mEq/L stands for?
|
Milli Equivalent per Litre.
|
|
|
In Na-K-ATPase how many ions of Na gets exchanged for how many K ions?
|
For every 2 K pumped in, 3 Na gets pumped out.
|
|
|
In resting membrane potential, what side of the cell is negative in relation to other side?
|
Inside of the cell is negative to outside - negative potential difference.
|
|
|
Who is called "father of physiology" ?
|
Claude Bernard. He was the first to define the term milieu intérieur (now known as homeostasis, a term coined by Walter Bradford Cannon).
|
|
|
Who was the first physiologist who used the term homeostasis?
|
Walter Cannon.
|
|
|
What is colligative properties?
|
Colligative properties are properties of solutions that depend on the number of molecules in a given volume of solvent and not on the properties/identity (e.g. size or mass) of the molecules. Colligative properties include: relative lowering of vapor pressure; elevation of boiling point; depression of freezing point and osmotic pressure.
|
|
|
What is Mole?
|
The mole is a unit of measurement for the amount of substance or chemical amount.he mole is defined as the amount of substance that contains as many elementary entities (e.g., atoms, molecules, ions, electrons) as there are atoms in 12 g of the isotope carbon-12 (12C).Thus, by definition, one mole of pure 12C has a mass of exactly 12 g.
|
|
|
How much is Avogadro number?
|

|
|
|
What is molarity?
|
A unit of concentration. The number of moles of solute per unit volume of solution, e.g. mol/L.
|
|
|
What is Osmole?
|
A unit of quantity. Contain Avogadro number of osmolyte particles. The amount of a substance that dissociates in solution to form one mole of osmotically active particles. It is independent of molecular weight and/or charge of solute particles.
|
|
|
Give an example to explain what is osmole.
|
A solution of 1 mol/L NaCl corresponds to an osmolarity of 2 osmol/L. The NaCl salt particle dissociates fully in water to become two separate particles: an Na+ ion and a Cl- ion. Therefore, each mole of NaCl becomes two osmoles in solution, one mole of Na+ and one mole of Cl-. Similarly, a solution of 1 mol/L CaCl2, gives a solution of 3 osmol/L (Ca2+ and 2 Cl-).
|
|
|
What is osmolarity?
|
The measurement of the activity of the solvent, the concentration of osmotically active particles in solution, which may be quantitatively expressed in osmoles of solute per liter of solution.
|
|
|
What is the formula for the osmolarity calculations?
|
Osmolarity = g x sum of molar concentrations of all osmolyte particles.
g - osmotic coefficient, the degree of dissociation of osmotic compounds in solution. |
|
|
How would you call a membrane that allows the passage of solvent but is impermeable to solute?
|
A semipermeable membrane.
|
|
|
What is the name of membrane that demonstrates varying degrees of permeability to solutes?
|
A selectively permeable membrane.
|
|
|
What is osmosis?
|
Osmosis is a flow of solvent particles from the area of higher solvent activity (lower osmolarity) to an area of lover solvent activity (higher osmolarity) across a semipermiable membrane. Osmosis is the movement of solvent molecules through a selectively-permeable membrane into a region of higher solute concentration, aiming to equalize the solute concentrations on the two sides.
|
|
|
What is osmotic pressure?
|
The necessary pressure to stop osmosis.
|
|
|
What can cause reverse osmosis?
|
The application of the hydrostatic pressure greater that osmotic pressure.
|
|
|
The Van't Hoff Equation.
|
π = gσCRT, where:
π - Osmotic pressure (atm) g - Osmotic coefficient σ - Reflection coefficient C - sum of all molar concentrations of all osmolyte particles (mol/L) R - gas constant (l.atm/mol/k) T - temperature (k - kelvin) |
|
|
What is osmotic pressure?
|
Suction pressure.
Osmotic pressure is the pressure that must be applied to a solution to prevent the inward flow of water across a semipermeable membrane. |
|
|
Tonicity?
|
Tonicity is a measure of the osmotic pressure (as defined by the water potential of the two solutions) of two solutions separated by a semipermeable membrane. It is commonly used when describing the response of cells immersed in an external solution.
|
|
|
Explain tonicity of the solutions - isotonic, hypertonic, pypotonic.
|
Tonicity of aqueous solutions (water with solutes, such as salt, dissolved in it) is based on cellular responses to that solution.
Solutions are isotonic if the cells or tissue neither shrink nor swell in response to immersion in that solution. Solutions are hypertonic if the cells or tissue shrink in response to immersion. Solutions are hypotonic if the cells or tissue swell in response to immersion. |
|
|
In which type of solution the effective pressure of ICF is the same as in ECF?
|
Isotonic solution.
|
|
|
Why exactly the saline 0.9% of NaCl is placed intravenously? Why now water of a saline of 7% of NaCl?
|
Because that is isotonic solution to the blood. If it would be less, the cells would burst, if that would be more, cells would shrink.
|
|
|
What happens if the ECF has higher effective osmotic pressure to ICF?
|
Hypertonic bathing solution. The cell volume decreases and cells shrink. E.g. salt water.
|
|
|
What happens if the ECF has lower effective osmotic pressure to ICF?
|
Hypotonic bathing solution. The cell volume increases and the cell burst. E.g. pure water.
|
|
|
Translate 1 mEq into mmol for monovalent, divalent, trivalent ions.
|
For monovalent ions, 1 meq = 1 mmol
For monovilent ions, 1 meq is equal to 1 mmol (Na ions) For divalent ions, 1 meq = 0.5 mmol (Ca ions) For trivalent ions, 1 meq » 0.333 mmol |
|
|
How many millimoles of Mg2+ would be present in a solution containing 0.8 milliequivalents?
|
0.4 millimoles
|
|
|
How do you get 1 mol/L solution of some substance?
|
Put 1 mole of the substance and add water till the volume reaches 1 Litre.
|
|
|
Calculate the osmolarity of 155 mmol/l of NaCl? g=0.93
|
Osmolarity = g x sum of all molar concentrations.
= 0.93 x (2 x 155) = 288.3 mOsm/l |
|
|
Explain the Fick's Law of simple diffusion.
|
Net flux = dAΔC/x, where
d - diffusion coefficient; A - surface area of the membrane; C - concentration gradient; x - thickness of the membrane. The rate of diffusion is proportional to the surface area of the membrane and also to the concentration gradient; and inversely proportional to the thickness of the membrane. |
|
|
What is the Einstein's equation says about the simple diffusion?
|
The time is proportional to the distance squired. So it takes four times as long to diffuse twice as far. Small changes in the distance of diffusion makes a big changes in the time of diffusion.
|
|
|
What allows the cell membrane to be permeable to the non lipid soluble substances such as sugars and amino acids?
|
Facilitated diffusion (integral proteins, e.g. ion channels).
|
|
|
Na-K-ATPase, Ca-ATPase, and H-K-ATPase are the wxamples of what type of transport?
|
Primary active transport.
|
|
|
What is the difference between primary and secondary active transport?
|
Primary active transport utilises metabolic energy directly, SAT - indirectly. In SAT one solute moves downhill coupled with uphill transport of second solute.
|
|
|
Name two types of secondary active transport.
|
Cotransport/symport and countertransport/antiport.
|
|
|
Give examples of cotransport.
|
Na-glucose; Na-amino acids; Na-K-2Cl.
|
|
|
Give examples of countertransport.
|
Ca-Na exchanger; Na-H exchanger; HCO3-Cl exchanger.
|
|
|
Name two types of passive transport.
|
Simple diffusion, facilitated diffusion.
|
|
|
Name types of active transport.
|
Primary active transport and secondary active transport (cotransport and dountertransport).
|

