![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
395 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Volatile acid |
Is CO2, created from metabolism of cells. CO2 combines with H2O to create bicarbonate. - Carbonic anhydrase catalyze the reversible reaction. |
|
|
|
Non-volatile acid |
An acid created in the body from other substances than CO2, and not excreted by the lungs. Fixed acid, like sulfuric and phosphoric Can be overdigested or produced |
|
|
|
ph of venous and arterial blood |
Arterial blood: pH=7.40 Venous blood: pH= 7.36 |
|
|
|
What regulated pH in the body? |
Buffers Lungs via CO2 elimination Kidneys via H+ secretion |
|
|
|
Buffers |
Prevent pH change when H+ ions are added or removed. Weak acid and conjugated base Weak base and conjugated acids |
|
|
|
Buffer base |
Bicarbonate and hemoglobin together 44-49 mmol/L |
|
|
|
Buffers in plasma |
Bicarbonate Protein, phosphate, ammonia |
|
|
|
Buffers in blood |
Bicarbonate, hemoglobin and organic phosphate |
|
|
|
Actual bicarbonate |
Blood bicarbonate measured at the actual pCO2 level |
|
|
|
Standard bicarbonate |
Blood bicarbonate level, when blood is saturated at 40 Hg mm pCO2 |
|
|
|
Base excess |
Means base deficit or sufficit which would be required to be added or removed in order to restore the blood pH to 7.4 at 40 Hgmm |
|
|
|
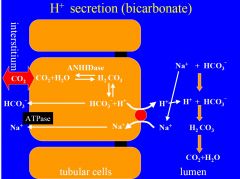
H+ secretion with bicarbonate |
Occurs in proximal tubule Causes netabsorption of filtered HCO3-, without net secretion of H+. 1.H+ and HCO3 is created in proximal tubule from CO2 and H2O. First H2CO3, then dissociate to H+ and HCO3. H+ will secreted to lumen via Na+-H exchange, while HCO3+- is reabsorbed. 2. In lumen the secreted H+ will combine with HCO3, and form H2CO3, which dissociates to CO2 and H2O by carbonic anhydrase. CO2 and H2O diffuse into cell and start cycle again. |
|
|
|
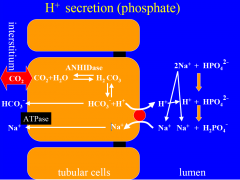
Excretion of H+ as H2PO4- |
Amount of H+ excreted as titratable acid will depend on amount of urinary buffer present, and pk. Result is net secretion of H+ and net reabsorption of HCO3. 1. H+ and HCO3 are produced in intercalated cell. H+ is secreted into lumen via H+-ATPase. HCO3- is reabsorbed into blood. In urine the H+ will join with filtered HPO4 and form H2PO4 H2PO4 is excreted. |
|
|
|
What increases the activity of H+-ATPase? |
Aldosterone |
|
|
|
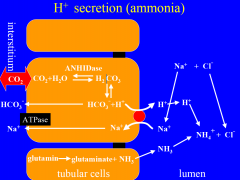
H+ secretion as NH4+ |
Amount of H+ which secreted is dependent on amount of NH3+ synthesized by renal cells and urine pH. NH3 is produced by renal cells from glutamine, and diffuses into lumen with it´s gradient. 1. H and HCO3 is produced in intercalated cells, and H´is secreted into lumen via H+-ATPase. In lumen H+ will bind with NH3, and form NH4. NH$ is excreted, while HCO3 is reabsorbed in blood. |
|
|
|
How does the pH of the tubular fluid affect the excretion of H+ via NH4? |
The lower the pH of the tubular fluid is, the greater the excretion of H+ via NH4 will be. Because when urine pH is low, there is more NH4 than NH3, which increases the diffusion of NH3 from the tubular cells.
|
|
|
|
How does hyperkalemia and hypokalemia affect NH3 synthesis? |
Hyperkalemia inhibits the NH3 synthesis Hypokalemia stimulates the NH3 synthesis |
|
|
|
What causes metabolic acidosis? |
Increased exogen acid intake increased endogen acid production Renal failure Excessive loss of alkalic fluid Hyperkalaemia - Inhibits NH3 synthesis |
|
|
|
What causes respiratory acidosis? |
Inhalation of high CO2 containing air Decreased respiratory surface - Pulmonary fluid - Inflammation Insufficient ventilation - Pneumothorax - Muscle weakness - Airway obstruction |
|
|
|
What causes metabolic alkalosis? |
Exogen intake of alkalic chemicals Loss of HCl acids Hypokalemia - increased NH3 synthesis |
|
|
|
What causes respiratory alkalosis?
|
CNS lesion
Artificial respiration Voluntary hyperventilation Panic attack Hypoxia |
|
|
|
BMR |
Basal metabolic rate is the minimal rate of energy expenditure per unit of time at rest. Standard criteria is applied. |
|
|
|
What is the base metabolic rate by organs? |
Liver - 1/4 Muscle - 1/4 Brain and heart - 1/4 Kidney and others - 1/4 |
|
|
|
What are the nutrient classes? |
Carbohydrates, proteins, lipids, vitamins, minerals and water |
|
|
|
What regulates food intake? |
Two hypothalamic centers regulate eating. 1. Feeding center - when stimulates it initiates feeding. 2. Satiety center - During stimulation causes cessation of eating, even if starved. Feeding center is always active, but satiety center can inhibit it. |
|
|
|
What causes the satiety center to inhibit the feeding center? |
May inhibit due to changes in blood composition - Glucostatic theory - Lipostatic theory - Aminostatic theory Other influences - Temperature -> high temp decreases appetite - Habit, hormones etc |
|
|
|
Homiothermic |
Warm blooded constant body temperature |
|
|
|
Heterothermic |
Constant/changing body temperature Hibernating |
|
|
|
Poikilothermic |
Cold blooded Changing body temperature |
|
|
|
What are some characteristics of core temperature? |
Temperature of internal organs Heat production Constant since it´s regulated Independent from ambient temperature Changes in narrow range: 36.5-37.1 |
|
|
|
What are some characteristics of surface temperature? |
Skin temperature Heat exchange - Loss and absorption Varying, depends on ambient temperature Changes in wide range Difference between distinct areas |
|
|
|
How is temperature regulated? |
Regulated via two systems 1. Chemical thermoregulation - Metabolism, muscular activity 2. Physical thermoregulation - Depends on ambient temperature - Heat loss - Heat absorption only above 34 C |
|
|
|
What influences internal temperature? |
Diurnal rhythm -> 1 C Menstrual cycle Seasonal changes Ambient temperature Work out Sleep Age Hormonal state Gender Emotional stress ingestion insulation |
|
|
|
What´s is the thermostat model? |
it´s the basis of thermoregulation. Set point -> temperature to achieve Error signal -> Regulation and negative feedback mechanism. - Cold signal -> decrease in heat loss, increase in heat production - Warm signal -> Increase i heat loss |
|
|
|
Ways of heat production |
1. Basal metabolism 2. Muscular activity -> shivering 3. Thyroxine and epinephrine 4. Temperature effect on cells |
|
|
|
Ways of heat loss |
1. Radiation - Loss of heat in form of infrared rays 2. Conduction -Transfer of heat between objects in contact 3. Convection - Transfer of heat by current 4. Evaporation - Thermal cooling by water |
|
|
|
Hypothermia |
Core temp below 35 C Mild, moderate and severe Heat production Increased heat loss in cold environment or newborns. Decreased heat production in malnutrition, elders, chronic diseases etc. |
|
|
|
Hyperthermia |
Core temperature above 38C Heat production > heat loss Overload of thermoregulation Heat loss inhibited by humid/warm, overclothing, impaired regulation, dehydration. Increased heat production due to increase muscle work, epilepsy, hyperthyreosis |
|
|
|
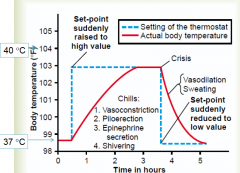
Pyrexia |
Fever Core temp above 38C 1. Subfebrility: 37-38 C 2. Moderate fever: 38-39 C 3. Severe fever: 39-40 C 4. Hyperpyrexia: above 40 C |
|
|
|
What´s the mechanism behind fever? |
|
|
|
|
What is the peripheral control mechanism for thermoregulation? |
1. Physical thermoregulation Processes of heat dissipation and heat absorption. Small changes in temperature -> heat loss is adjusted. 2. Chemical thermoregulation Heat producing process. - Larger and longer lasting changes in temperature. - Metabolism is adjusted. |
|
|
|
Non-evaporative heat exchange |
Heat loss or gain depending on temperature. Conduction Convection RAdiation |
|
|
|
Evaporative heat exchange |
Only heat loss Insensible perspiration -20-30% of heat loss - Can not be regulated directly - Surface of skin and airways Sensible perspiration - In high ambient temp, non-evaporative heat loss is not enough. Compensated by sweating. - Humidity influences the effectiveness of evaporation. |
|
|
|
Sweat |
Produced by sweat glands 1. Corpus - Almost isosmotic 2. Ductus - Leading to surface - Reabsorption of water and electrolyte -Aldosterone regulates Regulation - Neural -> sympathetic cholinergic - Humoral -> Bradykinin |
|
|
|
Shivering thermogenesis |
Heat producing process of muscles 1. Skeletal muscle tone increases - Increase heat prod by 50% 2. Shivering thermogenesis -> involuntary process - causes higher O2 consumption and heat production Maximum at 33-34 C core temp Stops at below 30 C |
|
|
|
Non-shivering thermogenesis |
Sympathetic stimulation -> NA and A increased - Metabolism of cells increase - total energy released increases - UCP proteins are important In newborns brown fat tissue Increased secretion of thyroid hormones |
|
|
|
Neutral temperature zone |
Temperature at which activity of heat producing and heat loss mechanisms are at a minimum. the person does not feel cold nor hot. naked: 27-30 C Dressed: 20-23 C |
|
|
|
What is the role of skin circulation? |
Blood flow through the skin -> skin temperature/heat loss Tissues are bad heat-conducters. Heat is transferred between core and surface via blood flow. Heat dissipation is depends on temperature gradient of core and skin. |
|
|
|
How is heat dissipation regulated in acral areas? |
Acral -> Extremities Sympathetic vasoconstrictor tone changes -> Regulates amount of blood flowing through skin, which in turn regulates the heat dissipation |
|
|
|
How is heat dissipation regulated in non-apical areas? |
Sympathetic vasoconstrictors have little influence. An active vasodilator will be used: Bradykinin |
|
|
|
What happens with the arteriovenous anastomoses during cold and warm weather? |
During cold weather they close, while they open during warm weather. Regulates blood flow through plexus of subcutis. |
|
|
|
Piloerection |
Goose bumps |
|
|
|
What type of thermoreceptors do we have? |
Peripheral thermoreceptors Core thermoreceptors Central thermoreceptors |
|
|
|
Peripheral thermoreceptors |
Found in skin Measures surface temp Thermoreceptors and thermal nociceptors |
|
|
|
Core thermoreceptors |
measure core temperature in deep body arterial blood walls of internal organs |
|
|
|
Central thermoreceptors
|
Core temperature of CNS Hypothalamus other brain regions Spinal cord |
|
|
|
Thermoreceptors |
Sensitive to cold or heat |
|
|
|
Thermal nociceptors |
Sensitive to temperature above 45 C or damaging cold. |
|
|
|
What kind of receptors do we find in skin? |
In the skin we find mostly cold thermoreceptors. Stimulation of these evoke reactions before a significant decrease in core temperature can happen. Shivering Sympathetic activation - Vasoconstriction, piloerection and increase in cell metabolism. |
|
|
|
Which part of the hypothalamus contribute to heat dissipation and heat conservation? |
Anterior hypothalamus -> heat dissipation Posterior hypothalamus -> Heat conservation |
|
|
|
Where do we find warmth sensing neurons? |
We find them in central thermoreceptors in the anterior hypothalamus. Activity of the heat dissipation area will increase based on the stimulation of these warmth receptors. |
|
|
|
Cold sensing neurons |
Will stimulate the posterior hypothalamus. - increased activity evoked by hypothalamic cold stimulation |
|
|
|
G proteins |
Guanosine triphosphate binding proteins, which couple with hormone receptors to adjacent effector molecule. - Used in adenylate cyclase and IP3 |
|
|
|
What are the subunits of G proteins? |
They have 3 subunits Alpha -> Can bind either GDP or GTP, when GDP is bound the g protein is inactive, when GTP it´s active. |
|
|
|
What the difference between Alpha I and Alpha S? |
G proteins can be stimulatory and inhibitory. Activity resides in the alpha subunit. Alpha S -> Stimulate Alpha I -> inhibit |
|
|
|
Describe the mechanism of adenylate cyclase in 7 steps |
1. Hormone binds to receptor in cell membrane. 2.GDP is releases and replaced by GTP in alfa subunit. 3. Activated g protein can either stimulate or inhibit adenylate cyclase. 4. Activated adenylate cyclase will catalyse ATP to cAMP. 5. cAMP will activate protein kinase A 6. cAMP is degraded to 5-AMP by phosphodiesterase |
|
|
|
Describe the IP3 mechanism in 3 steps |
1. Hormone will bind to receptors on cell membrane, and via G protein activate phospholipase C. 2. Phospholipase C liberates diacylglycerol and IP3 from membrane lipids. 3. IP3 mobilizes Ca2+ from ER 4. Ca2+ and diacylglycerol will together activate protein kinase C. |
|
|
|
What signals act through the guanylyl cyclase receptor? |
ANP or atrial natriuret peptide - GTP to cGMP Nitric oxide will act on cytosolic guanylyl cyclase - GTP to cGMP |
|
|
|
Receptor tyrosine kinases |
Hormone binds to extracellular part, intracellular side has intrinsic tyrosine kinase activity. Two types .> Monomers and dimers. - Monomers go through dimerization, then activate intrinsic tyrosine kinase. - Dimer will activate intrinsic tyrosine kinase. |
|
|
|
What type of tyrosine kinase is a receptor for insulin? |
Receptor tyrosine kinase dimer is a receptor for insulin. |
|
|
|
Tyrosine-associated receptors |
Mechanism of action of growth hormone. Intracellular side does not have tyrosine activity, but non-covalently associated with tyrosine kinase. Binding of growth hormone causes dimerization and activation of tyrosine kinase in associated protein, like JAK. |
|
|
|
Steroid hormone and thyroid hormone mechanism |
1. Will diffuse across the cell membrane, and bind to receptor. 2. Receptor complex will enter nucleus and dimerize. 3. Has transcription factors, which bind to steroid-responsive element of DNA. 4. Initiate DNA synthesis 5. Protein will have specific physiological actions |
|
|
|
What is the derivative of steroid hormones? |
Cholesterole |
|
|
|
What is the derivative of amine hormone synthesis? |
Tyrosine |
|
|
|
How is hormone secretion regulated? |
Negative feedback - Most common, self limiting - Hormone has biological actions which directly or indirectly inhibits further secretion. Positive feedback - Rare, self-reinforcing -> explosive - Hormone has biological actions which directly or indirectly lead to more secretion of that hormone. |
|
|
|
How are hormone receptors regulated? |
Down regulation of receptors - A hormone decreases the number or affinity of receptor for itself or an other hormone. Up regulation of receptors - A hormone increase the number or affinity of a receptor for itself or others. |
|
|
|
Example of down-regulating a hormone receptor? |
Progesterone will down regulate it´s own receptors and the receptors for estrogen. |
|
|
|
Example of up-regulating a hormone receptor? |
In the ovaries, estrogen will up regulates it´s own receptors and receptors of LH. |
|
|
|
What is the hypothalamic-hypophysial portal system? |
A system linking the anterior lobe of pituitary gland to the hypothalamus. Blood from hypothalamus, concentrated with hormones, will reach the anterior pituitary. Here it can either stimulate or inhibit secretion of anterior pituitary hormones. |
|
|
|
What is the posterior lobe derived from, and how does it secrete it´s hormones? |
Derived from neural tissue Pituitary hormones are synthesized within the nerve cell bodies, then packaged in granules and transported down the axons. Here they are released for circulation. |
|
|
|
What type of hormones are secreted from anterior pituitary? |
Growth hormones Adrenocorticotropic hormone Thyroid stimulating hormone Luteinizing hormone Follicle stimulating hormone Prolactin |
|
|
|
TSH, LH and FSH |
All secreted from anterior pituitary All from same glycoprotein family Each has alpha and beta subunit - Alpha is identical - Beta subunits are different, responsible for their individual biological activity |
|
|
|
Which hormones are derived from proopiomelanocortin? |
ATCH Melanocyte-stimulating hormone B-lipotropin B-endotropin |
|
|
|
Where are alpha-MSH and beta-MSH produced? |
Intermediary lobe |
|
|
|
What increases and decrease secretion of growth hormones? |
Secretion is increased by sleep stress, hormones related to puberty, starvation, exercise and hypoglemica. Secretion is decreased by somatostatin, somatomedins, obesity, hyperglemica and pregnancy |
|
|
|
What regulates growth hormones? |
1. Hypothalamic control -GHRH and somatostatin 2. Negative feedback control by somatomedins 3. Negative feedback control by GHRH and growth hormone |
|
|
|
Negative feedback control by GHRH and growth hormone - growth hormone regulation |
GHRH will inhibit it´s own secretion from hypothalamus Growth hormone will also inhibit it´s own secretion indirectly, by stimulating secretion of somatostatin |
|
|
|
Hypothalamic control - GHRH and somatostatin - growth hormone regulation |
GHRH will stimulate synthesis and secretion of growth hormones. Somatostatin will inhibit secretion of growth hormones, by blocking the response of the anterior pituitary to GHRH. |
|
|
|
Negative feedback control by somatomedins |
Somatomedins are produced when growth hormones act on target tissue. It will directly act on anterior pituitary gland, and act on hypothalamus by stimulating somatostatin secretion. |
|
|
|
Whats the direct action of growth hormones? |
Increase glucose uptake Increase lipolysis Increase proteins synthesis in muscle Increase production of IGF |
|
|
|
Whats the indirect action of growth hormones? |
Happens via IGF Increase protein synthesis in chondrocytes -> linear body growth Increase ps in muscle -> Lean body mass increase protein synthesis in organs -> size of organs |
|
|
|
Prolactin |
Major hormone responsible for lactogenesis secreted by the anterior pituitary Participates in estrogen and breast development. - Homologous to growth hormone |
|
|
|
What regulates prolactin? |
Hypothalamic control is done by dopamine and thyrotropin-releasing hormone (TRH). - Inhibited by dopamine - TRH increase prolactin secretion Negative feedback control -Proaction will inhibit itself by stimulating release of dopamine from hypothalamus |
|
|
|
Whats the symptoms of hyperthyrodism? |
Increased metabolic rate, heat production and CO. Weight loss Negative nitrogen balance Dyspnea Tremor Goiter |
|
|
|
What are the symptoms of hypothyrodism? |
Decreased metabolic rate, heat production, CO - Weight gain - Positive nitrogen balance - Hypoventilation - Mental slowness - Retardation |
|
|
|
How are the TSH levels affected by hyper and hypothyrodism? |
Hyperthyrodism - decreased b/c feedback inhibition of anterior pituitary by high thyroid hormone levels. Hypothryodism - Increased b/c decreased feedback on anterior pituitary. |
|
|
|
Gitrogens |
Compounds that lead to goiter - Attacking iodine uptake by the follicular cells - By inhibiting enzyme activity for iodination and coupling |
|
|
|
What does minimal concentration of T3 and T4 cause? |
Causes high concentration of TSH, -> cell proliferation -> enlargement of thyroid gland |
|
|
|
Synthesis of thyroid hormones |
Each step of is stimulated by TSH 1. Thyroglobulin into follicular lumen 2. Iodide pump or Na+-I cotransport 3. Oxidation of I- to I2 4. Organification of iodine 5. Coupling of MIT and DIT 6. Stimulating of thyroid cells by TSH 7. Binding of T3 and T4 8. Conversion of T4 to T3 |
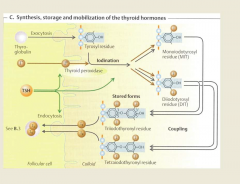
|
|
|
Wollf-Chaikoff effect |
High levels of I- will inhibit organification, and inhibit synthesis of thyroid hormones |
|
|
|
Regulation of thyroid hormone secretion |
1. Hypothalamic pituitary control - Done with TRH and TSH TRH secreted and stimulates secretion of TSH by anterior pituitary. TSH increase both synthesis and secretion of thyroid hormones via cAMP. T3 down-regulates TRH receptors, and will inhibit TSG secretion Thyroid stimulating immunoglobulins -IG bind and stimulate thyroid gland to secrete T3 and T4. |
|
|
|
T3 vs T4 thyroid hormone |
T3 is 3 times as potent as T4, and because of this target tissue will convert T4 to T3 |
|
|
|
Actions of thyroid hormones |
1. Growth 2. Development of central nervous system 3. Autonomic nervous system 4. Basal metabolic rate - Increased by thyroid hormone 4.Cardiovascular systems - Heart rate and stroke volume increased 5. Metabolic effects - Overall metabolism is increased |
|
|
|
synthesis of estrogen and progesterone |
1. Theca cells produce testosterone under LH stimulation.
2. Androstenedione will diffuse to nearby granulose cells containing hydroxysteroid dehydrogenase and aromatase. 3. Hydroxysteroid will convert androstenedione to testerone. 4. Aromatase will convert testosterone to 17B-estradiol |
|
|
|
Regulation of ovaries |
1. Hypothalamic control - GnRH - GnRH will stimulate anterior pituitary to secrete FSH and LH 2. Anterior lobe of pituitary - FSH and LH stimulate steroidogenesis, follicular development, ovulation and latinization 3. Negative and positive feedback -> done by estrogen and progesterone |
|
|
|
Negative and positive feedback control
of estrogen and progesterone on FSH and LH |
|
|
|
|
Whats the functions of estrogen? |
1. Negative and positive feedback effect on FSH and LH 2. Causes maturation and maintenance of cervix, uterus etc 3. Development of sex characteristics 4. Up-regulates estrogen, LH and progesterone receptors. 5. Proliferation of ovarian granulose cells 6. Maintains pregnancy 7. Stimulates prolactin secretion |
|
|
|
Functions of progesterone |
1. Negative feedback on LH and FSH 2. Maintains pregnancy 3. Secretory activity of uterus 4. Breast development |
|
|
|
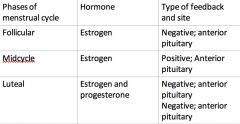
Follicular phase of menstrual cycle - Hormones |
Day 0 to 14 LH and FSH receptors are up-regulated in theca and granulose cells Estradiol levels rise FSH and LH are surpassed by negative feedback of estradiol. Progesterone levels are low |
|
|
|
Ovulation of menstrual cycles - hormones |
Burst of estradiol synthesis on the end of follicular phase -> cause positive feedback effect on secretion of FSH and LH -> LH surge. Ovulation occurs as result of estrogen induced LH surge Estrogen levels decrease just after ovulation |
|
|
|
Luteal phase of menstrual cycle - hormones |
Corpus luteum develops, synthesize estrogen and progesterone. If fertilization does not occur, corpus luteal will regress, causing abruptly decreasing estradiol and progesterone levels |
|
|
|
Menses of menstrual cycle - hormones |
Endometrium is sloughed because of the abrupt withdrawal of estradiol nd progesterone |
|
|
|
What rescues the corpus luteum from regression if there is fertilization? |
Human chorionic gonadotropin, produced by placenta |
|
|
|
Where is estradiol and progesterone produced during first trimester? |
Corpus luteum, which is stimulated by HCG |
|
|
|
Where is progesterone and estrogen produced in second trimester? |
Progesterone is produced by placenta Estrogen is produced by fetal adrenal gland and placenta. |
|
|
|
What increases the threshold for uterine contraction? |
Progesterone increases the threshold for uterine contraction, and closer to term the estrogen/progesterone ratio will increase. This causes the uterus to be more sensitive to contractile stimuli. |
|
|
|
Will lactation happen during pregnancy? |
NO, since estrogen and progesterone will block the action of prolactin on the breast. - Levels decrease, lactation occurs |
|
|
|
What is the regulatory function of prolactin? |
Prolactin will suppress ovulation as long as lactating happens. PL inhibits hypothalamic GnRH secretion PL inhibits action of GnRH, LH and FSH |
|
|
|
Synthesis of testosterone |
Testosterone is a major androgen, which is synthesized in leydig cells. LH increase testosterone synthesis by stimulating cholesterol desmolase. |
|
|
|
How is testosterone activated in sex organs? |
Sex organs have 5alpha - reductase which converts testosterone to it´s active form, dihydrotestosterone. |
|
|
|
How is testosterone regulated? |
1. Hypothalamic control - GnRH GnRH stimulate FSH and LH secretion 2. Anterior pituitary - FSH and LH - LH will act on leydig cells, promoting. - FSH will act on sertoli cells -> Inhibin 3. Negative feedback control - Inhibin and testosterone. - Testosterone inhibits secretion of LH, via GnRH and directly - Inhibin inhibits the secretion of FSH from anterior pituitary |
|
|
|
Action of testosterone |
1. Differentiation of vas deferens, epididymis and seminal vesicle 2. Pubertal growth spurt 3. Libido 4. Muscle mass 5. Growth of penis and seminal vesicle 6. Negative feedback on ap gland |
|
|
|
Action of dihydrotestosterone |
1. Differentiation between penis, scrotum and prostate 2. Hair, baldness 3. Sebaceous gland |
|
|
|
Androgen insensitivity disorder |
Caused by deficiency in androgen receptors in target tissue in males Actions of testosterone and dihydrotestosterone are absent Female genitalia, but no tract Testosterone levels are elevated -> no negative feedback |
|
|
|
Hormones in male and female sexual behavior |
Male -> testosterone Female -> testosterone and estrogens |
|
|
|
Oxytocin |
In male and female Produced in hypothalamus Stimulates the release of milk during breast-feeding. Released during physical touch - Increases sensitivity - Remain high after orgasm stress lowers oxytocin secretin |
|
|
|
Limbic system |
Associate with emotion, motivation and memory |
|
|
|
Dopamine during sexual arousal |
Released in pleasure center of limbic center Facilitates sexual arousal and response Stimulated by testosterone in both genders |
|
|
|
Serotonin |
Inhibits sexual activity Inhibits release of dopamine Antidepressants called SRRIs increase serotonin levels in brain - Side effect |
|
|
|
SRRI |
Antidepressant which increase serotonin levels in brain |
|
|
|
Adrenarche |
Beginning of androgen production of the adrenal gland Around 7-8 years |
|
|
|
The larche |
Begining of development of breast buds |
|
|
|
Pubarche |
Beginning of development of pubic hair growth |
|
|
|
Menarche |
Time of first menstruation |
|
|
|
Spermarche |
Beginning of sperm production |
|
|
|
Tanner´s rating of sexual maturity |
Tells us at which age sexual maturity a female or male is at, based on breast, testes, scrotum and pubic hair. |
|
|
|
Where is vasopressin ADH produced? |
Produced in hypothalamus, but stored in posterior hypophysis. |
|
|
|
What is the effect of vasopressin? |
Water retention - Increase expression of Aqua-2 channels in luminal, which facilitates water reabsorption Urea reabsorption Smooth muscle contraction -> vasoconstriction |
|
|
|
Vasopressin is regulated by |
Stimulated by - Increased plasma osmolality - Hypovolemia - Muscular work, pain, emotional stress Inhibited by - Decreased plasma osmolality - Hypervolemia - Alcohol |
|
|
|
Atrial natriuertric peptide or hormone |
Released from right atrium Factors which increase release - Wall stretch - Increased NaCl concentration Has effect on kidney and arterioles - Kidney -> increased GFR - Arterioles -> vasodilation |
|
|
|
Prostaglandins, and it´s derivatives |
Differ from endocrine hormones, since they are not produced at specific sites, but throughout the human body. Two derivatives Prostacyclin - Powerful vasodilator, inhibits blood platelets. - Role in inflammation Thromboxane - Strong vasoconstrictor, facilitate platelet aggregation |
|
|
|
effect of Gastrin |
Increase gastric H+ secretion Stimulates growth of gastric mucosa |
|
|
|
effect of CCK |
Stimulation of contraction of gallbladder and relaxation of sphincter of oddi. Increased pancreatic enzyme and HCO3- secretion Increased growth of exocrine pancreas and gallbladder Inhibits gastric emptying |
|
|
|
effect of Secretin |
Increase pancreatic HCO3- secretion Increase biliary HCO3- secretion Decrease gastric H+ secretion |
|
|
|
effect of GIP |
Increases insulin secretion Decrease gastric H+ secretion |
|
|
|
When does the breakdown of carbohydrates start? |
By the salivary galnds |
|
|
|
What inhibits gastrin? |
H+ concentration in lumen of stomach -> negative feedback Somatostatin Affected by VIP |
|
|
|
What stimulates secretin? |
Secretin is secreted for S cells in duodenum. Secretion is stimulated by high H+ levels in the duodenum HCO3- is used to neutralize |
|
|
|
What stimulates GIP secretion? |
GIP Is secreted by duodenum and jejenum. GIP is released in response to fat, protein, and carbohydrate |
|
|
|
Effect of motilin |
A candidate hormone Increases GI motility, and is involved in myoelectrical complexes. |
|
|
|
Hormone vs paracrines vs neurocrines |
Hormones -> secretion via portal system
Paracrine -> diffusion Neurocrines -> neuron gives off action potential |
|
|
|
Gi Paracrines |
Somatostatin and histamine |
|
|
|
Somatostatin in gi tract |
Secreted by cells throughout the GI tract in response to H+ in lumen Inhibits release of all GI hormones Inhibits gastric H+ secretion It´s secretion is inhibited by vagal stimulation |
|
|
|
Histamine in gi tract
|
Secreted by mast cells of the gastric mucosa Increases gastric H+ secretion directly, and by potentiating the effects of gastrin and vagal stimulation |
|
|
|
Neurocrines of Gi tract |
Synthesized in neurons of GI tract, moved via axons and released by action potential in the nerves. Diffuse across to target cell VIP, GRP and enkephalins |
|
|
|
VIP |
Produces relaxation of GI smooth muscle Stimulates pancreatic HCO3 secretion and inhibits gastric H+ secretion. |
Resembles secretin |
|
|
GRP |
Released from vagus nerve Stimulates gastrin release |
|
|
|
Enkephalins
|
Stimulate contraction of GI smooth muscle cells Inhibit intestinal secretion of fluid and electrolytes. |
|
|
|
How is salivary secretion regulated? |
Parasympathetic: High flow rate, watery Sympathetic: decreases salivary secretion, lower flow rate and larger viscous |
|
|
|
How much is the gastric secretion and salivary secretion per day? Pancreatic juice? |
Gastric 2.5-3.5 liter/day salivary: 1-1.5 liter/day Pancreatic juice: 2liters/day |
|
|
|
What increases HCl secretion? |
Histamine, gastrin, vagus nerve, food |
|
|
|
What inhibits HCl secretion?
|
negative feedback Fat containing food GIP Somatostatin Secretin CCK |
|
|
|
Receptive relaxation |
A vagovagal reflex which is initiated by distention of stomach. CCK participates, increasing the distensibility of stomach No efferent input via vagus nerve |
|
|
|
What inhibits gastric emptying? |
Fat inhibits gastric emptying by stimulating CCK release. H+ in the duodenum will also inhibit gastric emptying |
|
|
|
When is the rate of gastric emptying the fastest? |
When the content of the stomach is isotonic, it´s slowed if the content is hypertonic or isotonic. |
|
|
|
Gastroileal reflex |
Reflex mediated by extrinsic ANS, and gastrin. Presence of food in stomach triggers peristalsis in ileum and relaxation of ilieoceacal sphincter To empty the colon |
|
|
|
Gastrocolic reflex |
Presence of food in stomach increases motility of the colon and increases the frequency of mass movements Parasymp when stomach is stretched by food. CCK and gastrin |
|
|
|
What is the major characteristics of gastric secretion? |
HCl, pepsinogen, intrinsic facotor |
|
|
|
What is the major characteristics of pancreatic secretion? |
HCO3- Isotonic Pancreatic lipase, amylase and proteases |
|
|
|
What are the two major functions of bile? |
1. Digestive/secretory - bile acids - Detergents - Binding of colipase to micells - Activate lipid degrading enzymes 2. Not digestive/excretory - Excretion of cholesterin - Excretion of bile pigments - bilirubin |
|
|
|
What is bile secretion stimulated by? |
CCK Parasympathetic nervous system - Vagus nerve |
|
|
|
What is pancreatic secretion stimulated by?
|
Secretin CCK parasympathetic nervous system - Vagus nerve |
|
|
|
What is gastric secretion stimulates by |
Gastrin Parasympathetic nervous system - Vagus nerve Histamine |
|
|
|
How does the pancreatic composition change due to flow rate? |
At low flow rate the isotonic fluid is mostly consistent of Na and Cl. At high flow rates the isotonic fluid is mostly consistent of NA and HCO3 |
|
|
|
What are the different biles? |
Liver bile is C-bile. - Produced by hepatocytes. Gallbladder bile is B-bile. - Concentrated and stored Duodenum bile is a-bile - Released |
|
|
|
What does bile contain? |
Bile contains water, ions, lectinine, cholesterole, bile acids, salts and pigments, proteins, organic metabolites. - bile pigments - bilirubin Bile does not contain enzymes |
|
|
|
What´s the function of bile salts? |
Lipid emulisification Lipase-activation |
|
|
|
Primary bile salts |
Synthesized from cholestrole by hepatocytes. - Steroids Convert to secondary in ileum. |
|
|
|
Recirculation of bile acids to liver |
Bile acids are reabsorbed by secondary active transporters like Na´-bile acid cotrasnporter. They are transported/recycled to the liver when they reach the terminal ileum, |
|
|
|
Secretin stimulation on pancreas |
Secretin stimulates ductal cells, increasing HCO3 secretion. Second messenger is cAMP |
|
|
|
CCK stimulation on pancreatic secretion
|
CKK acts on acinar cells, to increase enzymatic stimulation IP3 as 2nd messenger |
|
|
|
ACh stimulation on pancreatic secretion |
ACh stimulates enzymes secretion by acinar cells. |
|
|
|
Vagal stimulation of stomach |
Will increase H+ secretion by direct and indirect pathway. - Innervating parietal cells to stimulate H+ - Innervate G cells to secrete gastrin |
|
|
|
Why doesn't´t atropine block H+ secretion completely in stomach? |
Because it only blocks H+ secretion from the direct pathway. But does not inhibit the indirect pathway |
|
|
|
What does vagotomy to gastric H+ stimulation? |
It eliminates it both directly and indireclty |
|
|
|
Gastrin stimulation of stomach |
Released in response to eating a meal Stimulates H+ secretion via CCK receptors on parietal cells. Second messenger is IP3 and Ca2+ Gastrin also simulates histamine secretion, which stimulates H+ secretion. |
|
|
|
Histamine stimulation of stomach |
Released from eCL cells, and diffuse to nearby parietal cells. Activates H2 receptors on parietal cell membrane. cAMP as second messenger |
|
|
|
Inhibition of gastric H+ secretion |
Negative feedback Low pH in stomach - inhibits gastrin secretin -> inhibits H+ secretion Somatostatin - Inhibits H+ secretion in two ways- direct and indirect Prostaglandins - Decrease cAMP levels |
|
|
|
How are carbohydrates digested? |
First salivary amylase starts the starch digestion Pancreatic amylase digest starch to oligosaccharides. Oligosaccharides are hydrolyzed by brush border enzymes. Glucose is transported via Na+ into capillaries. |
|
|
|
How is carbohydrates absorbed? |
Only as monosaccharides Glucose and galactose in two steps via transporter. Mannose and pentose via diffusion Fructose uses Glut-5 to facilitate diffusion |
|
|
|
How is fat digested? |
Digestion starts with salivary lipase and gastric lipase. Lipids in duodenum break up fat droplets and form micelles. Pancreatic lipase and colipase hydrolyze triglycerids to form free fatty acids and monoglycerides. |
|
|
|
Where is fat absorbed?
|
Lipids are absorbed in lower duodenum and upper jejunum. - Either directly or by first forming lipoproteins |
|
|
|
How is protein digested? |
starts in stomach, as pepsin digest proteins to form polypeptides. Pancreatic jucie and small intestinal fluid continue the digestion - Endopeptideoases cleave interior of the polypeptide - Exopeptidases cleve the ends of polypeptide |
|
|
|
In which forms are protein digested? |
Protein is digested in two forms Di-and tripeptides Aminoacids |
|
|
|
Function of kidneys |
Regulation of fluid and electrolyte balance - Volume regulation and pH regulation - Maintaining isoiona and isosmose Excretion of metabolic products and toxins Production of enzymes - Renin etc Elimination of insulin |
|
|
|
GFR |
Glomerular filtration rate GFR= Amount excreted/time*plasma inulin concentration* urinary inulin concentration GFR= Kf*net filtration pressure *KF= capillary filtration coefficient |
|
|
|
What is the filtered fluid made up of? |
Glomerular capillaries are almost impermeable to proteins, so filtered fluid is mostly made up of salts and organic molecules. Similar to plasma - ions, little organic molecules - Poor in proteins |
|
|
|
What determines GFR? |
1. Balance of hydrostatic and colloid forces, which act on the glomerular capillary membrane - Sum of these become net filtration pressure 2. Capillary filtration coefficient - KF - Product of permeability and filtering surface area. |
|
|
|
What will affect net filtration pressure? |
1. Hydrostatic pressure inside glomerular capillaries - Promotes filtration 2. Hydrostatic pressure in bowman´s capsule - opposes filtration 3. Colloid osmotic pressure in glomerular - opposes filtration 4. Colloid osmotic pressure in bowman´s capsule - promotes filtration |
|
|
|
What promotes filtration?
|
High colloid osmotic pressure in bowman´s capsule and high hydrostatic pressure in glomerular capillaries. |
|
|
|
What opposes filtration? |
High osmotic colloid pressure in glomerular capillaries and high hydrostatic pressure in bowman´s capsule. |
|
|
|
Filtration factor |
GFR/renal plasma flow |
|
|
|
How does the molecules characteristics change its filtration rate? |
Molecular weight increases filterability decreases. Negatively charged molecules are harder to filtrate than the positively charged molecules of same size |
|
|
|
Renal oxygen consumption |
Oxygen delivered to the kidneys will far exceeds its needs. Renal oxygen consumption varies proportionally to the renal tubular sodium reabsorption. - Which again is related to GFR. If GFR decreases, renal reabsorption will decrease, and oxygen consumption will decrease |
|
|
|
Clearance of PAH to calculate RPF |
We can use PAH to calculate the renal plasma flow. No substance is completely cleared from plasma, but 90% of PAH is. Total renal plasma=PAH clearance/PAH extraction ratio. PAH extraction ratio is calculated based on difference of PAH in arterial and venous renal vessels, divided by renal arterial PAH concentration |
|
|
|
FF |
Filtration factor The fraction of plasma which filtrates through the glomerular mambrane. FF=GFR/RPF |
|
|
|
Whats the function of the auto regulation of kidneys? |
The function is to maintain a constant GFR, and allow for precise control of renal excretion of water and solutes |
|
|
|
How does diameter changes in efferent and afferent arteries change GFR? |
Vasodilation in afferent increases GFR Vasoconstriction in afferent decreases GFR Vasodilation in efferent decreases GFR Vasoconstriction in efferent increases GFR |
|
|
|
How is RBF regulated |
Vasoconstriction of renal arterioles-> decrease RBF. Done by angiotensin 2 and sympa. Angiotensin-converting enzyme or ACE inhibitors will dilate efferent arterioles, producing decreased GFR. Vasodilation of renal arterioles lead to increased RBF. - Prostaglandings E and I, bradykinin, nitric oxide and dopamine |
|
|
|
How is GFR protected during low concentrations? |
At low concentrations, angiotensin II will constrict efferent arterioles, to increase the GFR |
|
|
|
What vasodilates renal arterioles? |
Prostaglandins E2 and I2, bradykinin, nitric oxide and dopamine. causes an increased RBF |
|
|
|
How does atrial natriuretic peptide or ANP effect RBF? |
It will cause vasodilation of afferent arterioles, and to a lesser extent, vasoconstriction of efferent arterioles. ANP increases RBF |
|
|
|
What are the mechanisms for autoregulation? |
Myogenic mechanism
- Renal afferent arterioles contract in response to stretch. Meaning when increased arterial pressure, it will contract, which increases resistant, and maintains a constant blood flow. Tubuloglomerular feedback - Increased arteriole pressure leads to increased delivery of fluid to macula densa. Macula densa senses the increased load, and causes constriction of nearby afferent arterioles. |
|
|
|
RAAS |
Renin-angiotensin-aldosterone system
Regulates blood volume, bp, cardiac and vascular function, regulation of water and salt intake. |
|
|
|
Renin |
Protelytic enzyme which transforms ATG to AT in liver. Stimulated by BP decrease, bleeding and dehydration, sympa from B1. Inhibited by negative feedback from AT-II or aldosterone |
|
|
|
Angiotensin I |
Converted from angiotensinogen to angiotensin I. Has no physiological activity, but is activated by angiotensin converting enzyme. AT-I to AT-II ACE is produced in pulmonary capillaries |
|
|
|
ACE |
Angiotensin converting enzyme Proteolytic enzyme, which converts AT-I to AT-II Produced in the pulmonary capillaries |
|
|
|
Angiotensin II |
Active form, mostly produced in pulmonary capillaries. Effect of AT Vasoconstriction - Causing BP to increase, and RBF and GFR to decrease Ion-secreting effects in kidney - Sodium reabsorption is increased Salt appetite and thirst increases Secretion of aldosterone |
|
|
|
Aldosterone |
Aldosterone is the steroid which is produced in response to angiotensin II. Effects kidney, sweat and salivation |
|
|
|
What does aldosterone effect? |
Effect on kidneys - Na/k+ ratio increases in blood and decreases in tubular fluid. -NaCl retention - Water retention - K+ and H+ secretion Sweat -> Na/K ratio increases in blood, down in sweat Salivation -> Na/K ratio increases in blood, down in sweat |
|
|
|
Reabsorption and secretion rate |
Reabsorption and secretion rate is the difference between what's filtered across the glomerular membrane and what's excreted with urine |
|
|
|
Excretion rate |
excretion rate= Volume*urine concentration |
|
|
|
Filtrated load |
Fl= GFR* plasma concentration |
|
|
|
Reabsorption rate |
Filtered load - excretion rate |
|
|
|
Secretion rate
|
Excretion rate - filtered load |
|
|
|
If filtered load is greater than excretion rate.. |
Net absorption of the given substance has occurred |
|
|
|
If excretion rate is greater than filtered rate.. |
The net secretion of that given substance has occurred |
|
|
|
Filtered rate of glucose? |
Filtration rate of glucose will increase directly proportional to the plasma glucose concentration
|
|
|
|
Transport of glucose |
Glucose will be reabsorbed by Na-glucose cotransporters in proximal tubule. There are limited amounts of these transports. Concentrations below 250 mg/dl all glucose will be reabsorbed. Concentrations above 350mg/dl -> carriers are saturated, and glucose reabsorption will not increase |
|
|
|
Transport maximum for glucose? |
Happens when glucose concentration is above 350 mg/dl Transport maximum for glucose is reached when all the Na-glucose cotransporters are saturated. Plasma levels below 250mg/dl all glucose will be reabsorbed |
|
|
|
Splay |
Region of glucose curve between threshold and transport maximum. Represents the excretion of glucose in urine before saturation of reabsorption has been achieved |
|
|
|
PAH's filtered load |
PAH´s filtered load increase directly proportional to plasma concentration. Happens in proximal tubule via carriers. Carriers becomes saturated at one point, which causes no further increase in secretion rate. When calculating RPF with clearance of PAH, we need to keep the plasma concentration below transport maximum |
|
|
|
Substances with highest clearance rate |
Those which both filtered across the glomerular capillaries, and are secreted from the peritubular into the urine |
|
|
|
Substances with lowest clearance rate |
Those substances that are not filtered, or are filtered bu reabsorebd by peritubular capillary blood, like Na, glucose, aa etc. |
|
|
|
Substances with equal clearance to GFR |
Inulin Glomerular markers, these are freely filtrated and not reabsorbed or secreted. |
|
|
|
What are the ways of tubular transport? |
Transcellular - Through the tubular cell - Active or passive - Pumps, carriers or channels Paracellular - Among tubular cells - Always passive transport according to the electrochemical gradient |
|
|
|
Transport characteristics of thin descending loop of Henle´s |
Very permeable to water |
|
|
|
Transport characteristics of thick ascending loop of Henle´s |
25% of filtered load is reabsorbed - Na, Cl, K, HCO3, Ca, Mg - Secretion of H+ - not permeable to H2O |
|
|
|
Transport characteristics of early distal tubules |
5% filtered load is resorbed NaCl is reabsorbed Ca++. Na+, Cl-, K+ and Mg -Active reabsorption Not permeable to H2O Not very permeable to urea Contains macula densa cells |
|
|
|
Transport characteristics of late distal tubules and collecting tubules |
Aquaporin 2 channels facilitate water reabsorption - Stimulated by ADH Na-Cl resorption - Aldosterone Not very permeable to urea Tubules principal cells secrete K+ Tubules intercalated cells secrete H+ |
|
|
|
Transport characteristics of medullar collecting ducts |
Reabsorption of Na, Cl Permeable to urea, HCO3 and H2O - H2O when ADH is present Absorption of H+ |
|
|
|
Angiotensin II's effect on tubular reabsorption |
Effects proximal tubule, thick ascending, distal tubule and collecting tubule. NaCl and H2O reabsorption increases H secretion increases |
|
|
|
Antidiuretic hormone's effect on tubular reabsorption |
Distal tubule and collecting tubule and duct. H2O reabsorption increases |
|
|
|
Atrial naturetic peptide |
Distal tubule and collecting tubule and duct Decrease in NaCl reabsorption |
|
|
|
Parathyroid hormone |
Proximal tubule, ascending loop of henle´s PO4 reabsorption decreases Ca++ reabsorption increases |
|
|
|
What stimulates ADH secretion? |
Increased osmolarity Decreased blood volume Decreased blood pressure Stimuli from cerebral cortex, angiotensin II, nausea, nicotine, morphine |
|
|
|
What decreases ADH secretion? |
Decreased osmolarity Increased blood volume Increased blood pressure |
|
|
|
Antidiuretic hormone |
Synthesized in hypothalamus, released by posterior pituitary gland and functions on kidney Stimulates H2O reabsorption and thirst |
|
|
|
How is diluted urine formed? |
Caused by decreased ADH release, which reduces the water permeability in the distal and collecting tubules. |
|
|
|
How is concentrated urine formed? |
Produced when ADH levels are high, due to water deprivation, volume deprivation or SIADH. Continued electrolyte reabsorption Increased water reabsorption |
|
|
|
What causes the buildup of solute in the renal medulla? |
Countercurrent multiplier Active transport of ions from thick ascending loop to interstitium. Active transport of ions from medullary collecting ducts to interstitium Passive diffusion of urea from medullary collecting ducts into interstitium Diffusion of only small amounts of water into medullary interstitium |
|
|
|
What is the result of countercurrent multiplier? |
More solute than water is added to renal medulla. Fluid in the ascending loop is diluted Most of water reabsorption occurs in cortex |
|
|
|
Central diabetes insipidus |
Failure to produce ADH |
|
|
|
Nephrogenic diabetes insipidus |
Failure to respond to ADH |
|
|
|
What is the range of osmolarity of urine? |
50 mOsm/kg water to 1300 mOsm/kg water |
|
|
|
What is the effect of ANP and what stimulates ANP? |
ANP is stimulated by atrial pressure and high NaCl levels
Increase in GFR, decrease in Na+ reabsorption |
|
|
|
What is the effect and stimuli of PTH? |
Decrease in calcium concentration in plasma stimulates it. Decreases phosphate reabsorption Increases Ca reabsorption |
|
|
|
What is micturition? |
It´s the process of emptying the urinary bladder. 1. Bladder fills until tension above threshold 2. Elicits a nervous reflex, which empties bladder. |
|
|
|
Addition of isotonic fluids |
Unchanged ECF osmolarity 1. Will cause a volume change in ECF, but no change in osmolarity since fluid is isotnoic 2. Plasma protein concentration and hematocrit will decrease, as it dilutes. RBC will not shrink 3. Arterial blood pressure will increase, since volume is added. |
|
|
|
Addition of NaCl |
1. ECF volume will change, and osmolarity will increase. Causing a shift from ICF to ECF, to equal out the osmolarity.3 2. ECF volume will increase, ICF decrease 3. Plasma and hematocrit levels will decrease. |
|
|
|
Loss of water |
1. ECF volume will change, and osmolarity will change. Shift of water from ICF to ECF 2. Plasma will increase, but no change happen due to the hematocrit, since water will shift out of RBC |
|
|
|
What happens with carbohydrate in liver? |
Almost al monosaccharides which are absorbed from the GI tract are converted to glucose in the liver. Glycogenolysis, glycogen synthesis, glycolysis, gluconeogenesis, pentose-phosphate pathway |
|
|
|
What metabolic functions does the liver have? |
Carbohydrate metabolism Protein metabolism Lipid metabolism |
|
|
|
What are the vasodilators produced by endothelium? |
Endothelium derived relaxing factor - ERDF Nitric oxide Prostacyclin |
|
|
|
Juxtaglomerular apparatus regulates what? |
Glomerular filtration |
|
|
|
Where does most of the sodium and water reabsorption occur? |
Proximal convoluted tubule |
|
|
|
When can substances move from blood to tubular fluid? |
During glomerular filtration and tubular secretion |
|
|
|
Is the cortical or medullary blood flow of the kidney highest? |
The cortical |
|
|
|
How is the lining of the glomerular capillaries? |
Highly fenestrated Allow free passage of solutes, ions etc |
|
|
|
Mesengial cell nertwork |
Provides structural support for the capillaries Can contract, reducing the glomerular surface area, and thereby the filtration rate. |
|
|
|
If the pH is below 7.4, and the Pco2 is below 40 mmHg what is most likely? |
Metabolic acidosis |
|
|
|
If the pH is below 7.4, and the Pco2 is above 45 mmHg, what is most likely?
|
Respiratory acidosis |
|
|
|
What will distention of stomach cause? |
Increased acid secretion by parietal cells |
|
|
|
why is regulating hormone receptor´s important? |
Determines the pathway of signal transduction, extent of endocrine response, responsible for first level of amplification of after hormone release
|
|
|
|
What hypothalamic hormones regulating anterior pituitary secretion as stimulatory and which are inhibiters? |
TRH, ADH, Ocytocin, Adrenocorticotropic hormone and PRL are stimulatory Somatostatin and dopamine are inhibitors |
|
|
|
What does normal secretion of estrogen in the ovaries require? |
Both FSH andLH And involves active granulose and theca cells |
|
|
|
Enterogastric reflex |
signals from the colon and small intestine to inhibit stomach motility and stomach secretion |
|
|
|
Colonoileal reflex |
Reflexes from colon to inhibit emptying of ileal contents into the colon
|
|
|
|
What can cause the reverse enterogastric reflex? |
Presence of food in small intestine Distending small bowel Presence of acid in duodenum Presence of protein breakdown products |
|
|
|
Acetylcholineesterase or AChE |
An enzyme which degrades ACh to acetyl CoA and choline on the muscle end of the plate. This causes the inactivation of a neurotransmitter |
|
|
|
What is the resting membrane potential of a nerve cell, and what causes it |
Resting membrane potential is at -70mV. This is because the conductance of K+ is high, which cause the membrane potential to be closed to K+ equilibrium potential. At rest Na+ channels will be closed |
|
|
|
What causes the upstroke of the action potential in a nerve cell? |
It´s caused by a inward current, which depolarized the membrane. This depolarization causes the voltage-gated Na+ channels to rapidly open, and increasing the Na+ conductance. This causes the membrane potential to move towards Na+ equilibrium potential at +65mV. Inward current of Na+ |
|
|
|
Tetrodotoxin and lidocaine |
Will block voltage sensitive Na channels, and abolish action potential |
|
|
|
Overshoot
|
At the peak of the action potential, when membrane potential is positive |
|
|
|
Undershoot |
After the repolarization the K+ conductance will remain higher than it is in resting, causing the membrane potential to be closer to the K+ equilibrium potential. |
|
|
|
Repolarization of a nerve action potential |
Depolarization will also close the inactivation gates of the Na+ channels, but much slower than it opens then the activation gates. |
|
|
|
pancreatic acinar cells vs ductal cells |
Pancreatic acinar cells produce enzymes, while ductal cells secrete bicarbonate and enzymes of the acinar cells. |
|
|
|
Which enzymes are part of the intestinal epithelial cell secretion - brush border? |
Maltase, aminopeptidase, lactase and sucrose |
|
|
|
How are triglycerides transported through the lymphatic vessels? |
In chylomicrons |
|
|
|
What causes fat cells to be more sensitive to epinephrine?
|
Growth hormones |
|
|
|
What increases the rate of testosterone synthesis in leydig cells?
|
LH |
|
|
|
What hormone released during pregnancy will reach it´s maximal level during first trimester? |
hCG |
|
|
|
What elicts the Hering-Breuer reflex?
|
Stretch of alveolar wall to proper extent Stimulation of mechanoreceptors within alveolar wall Forced inspiration Hyperventilation |
|
|
|
Carotid sinus reflex |
Caused by stretch in arterial wall - Decrease in blood pressure and HR - Uses baroreceptors |
|
|
|
Baroreceptors |
Fast, neural mechanism Negative feedback system, which is responsible for minute to minute regulation of arterial blood pressure. Stretch receptors. |
|
|
|
What is an example of the baroreceptors mechanism?
|
Valsalva maneuver Expiring towards closed glottis Causes increased intrathoracic pressure, which causes decreased venous return, which causes decreased arterial pressure sensed by baroreceptors. which causes increased heart rate. |
|
|
|
Depressor reflex |
Stretch of arterial wall Causes decrease in BP and HR Baroreceptors |
|
|
|
Bainbridge reflex |
Caused by increase in central venous pressure -> Stretch of atrial wall which is sensed by baroreceptors Increase in HR and BP |
|
|
|
Cushing reflex |
Caused by increased intracranial pressure sensed by intracranial baroreceptors Decrease in HP and increase in BP |
|
|
|
Atrialrenal reflex |
Stretch of atrial wall Baroreceptors in right atrium Increased urination |
|
|
|
Oculo-cardialreflex |
Compression of eyeball Decrease in heart rate |
|
|
|
Bezold-Jarisch reflex |
Caused by stretch of ventricular wall Baroreceptors and pain receptors Decrease in HR and BP |
|
|
|
Chemoreceptors |
two types peripheral and central They are sensitive to pH of cerebrospinal fluid. Decrease in pH of the CSF will produce increased breathing rate |
|
|
|
Chemoreflex - hypoxia |
Increase in heart rate, TPR and BP
chemoreceptors |
|
|
|
Lovenreflex |
Painful stimulus Causes increase in BP, HR and TPR Pain receptors |
|
|
|
Goltz reflex |
Mechanical stimulus of abdomen Causes decrease in Heart rate Abdominal mechanoreceptors |
|
|
|
How does decreased compliance effect the pulse pressure? |
It increases the pulse pressure |
|
|
|
Chronotropic effect |
Produce changes in heart rate |
|
|
|
Dromotropic effect |
Causes changes in conduction velocity, in AV node primarily |
|
|
|
Negative chronotropic effect |
Causes a decreased heart rate Neuotransmitter is acetylcholine which acts on muscarinic receptors |
|
|
|
Postive chronotropic effect |
Causes increased heart rate By norepinephrine which acts on B1 receptors. |
|
|
|
inotropic |
Contractibility of the cardaic msucle It´s intrinsic ability to develop force at given muscle length Related to intercellular Ca2+ concentration |
|
|
|
Frank-starling relationship |
Describes the effect that causes increased cardiac output and stroke volume, in response to increased venous return, or end-diastolic volume. |
|
|
|
Transmular pressure |
Transmural pressure is alveolar pressure minus intrapleural pressure |
|
|
|
What is the intrapleural pressure during inspiration and expiration? |
During inspiration the intrapleural pressure is negative, and the lungs expand, and lung volume increase. During expiration the intrapleural pressure is positive, the lungs collapse and lung volume decrease |
|
|
|
When's the compliance of lungs the highest? |
During the middle rang of pressures. At high expanding pressures, compliance is lowest, flattening the curve. |
|
|
|
Coronary circulation |
Controlled almost entirely by local metabolic factors mediated by sympathetic nerves Most important local metabolic factors are hypoxia and adenosine |
|
|
|
Cerebral circulation |
Controlled mainly by local metabolic factors Exhibits active and reactive hyperemia Most important local vasodilator is CO2 Sympathetic nerves play a minor role |
|
|
|
What is the structure of the walls of true capillaries? |
No smooth muscle, consisting only of one single layer of endothelial cells, which are surrounded by basement membrane |
|
|
|
Polycythemia |
Increased RBC count Higher viscosity, Viscosity is proportional with resistance |
|
|
|
Vasopressin is also called |
Antidiuretic hormone |
|
|
|
What's the result of hypoventilation? |
Respiratory acidosis |
|
|
|
Where does the countercurrent multiplayer take place? |
Loop of Henle´s |
|
|
|
What are the major factors to the countercurrent multiplayer |
Takes place in loop of Henle's 1. Active transport of ions into the medullary interstitial from the ascending loop of Henle´s 2. Active transport of ions from the collecting ducts to the interstitial. 3. Diffusion of urea from collecting ducts 4. Diffusion of only small amounts of water into the medullary interstitium |
|
|
|
During breathing, how is the intrapleural pressure in relation to the atmospheric pressure? |
The intrapleural pressure is always smaller than the atmospheric pressure. Always negative |
|
|
|
During inspiration, how is the intrapulmonary pressure in relation to the atmospheric? |
the intrapulmonary or the alveolar pressure has to be smaller than the atmospheric pressure during inspiration |
|
|
|
During expiration, how is the intrapulmonary pressure in relation to the atmospheric
|
During expiration the intrapulmonary pressure has to be higher than the atmospheric pressure. |
|
|
|
During expiration, how is the intrapleural pressure in relation to the atmospheric? |
Always negative, but during expiration it becomes more positive, but still negative |
|
|
|
During inspiration, how is the intrapleural pressure in relation to the atmospheric? |
During inspiration, the intrapleural pressure will decrease. To -6 |
|
|
|
where in a neuron do we find voltage gated Na+ channels? |
In the Ranvier nodes, between the myelin sheath |
|
|
|
What causes the non-peaky AP in SA node and AV node, but Peaky or sharp AP in His bundle, Purkinje fibers, ventricle and Atrium?
|
Those that have a peaky AP, will have fast sodium channels opening during phase0. SA and AV nodes have no fast sodium channels open during phase 0. |
|
|
|
What are the pacemaker currents? SA and AV node |
T-Type calcium channel in Nonselective cation current in or funny channel |
|
|
|
When do funny currents or no selective cation current open?
|
opens during hyperpolarization |
|
|
|
What is the resting potential in SA node, and how does this affect the fast sodium channels? |
The resting potential is around -55 millivolt This causes the fast sodium channels to already be inactivated. |
|
|
|
What channels open during phase 0, 1, 2, 3 and 4 in a working muscle cell? |
0. Fast sodium current in
1. L-type calcium in, transient potassium current out, chloride in 2. L- type calcium current in 3. Late potassium current out 4. Inward rectified potassium current |
|
|
|
How long is the refractory period? |
200 ms Last from phase 0 until the middle of phase 3 |
|
|
|
Where is voltage gated K channels found on the general neurone? |
Found on thee input component and the conductive component of the neuron Dendrit and soma, and axon |
|
|
|
Where is the Ligand gated ion channels found on the general neuron? |
On the input component Dendrites and soma |
|
|
|
where is the voltage gated Ca2+ channels found on the general neuron?
|
At the output component - Synaptic terminal |
|
|
|
What are the general characteristics of a chemical synapse? |
1. Action potential in presynaptic cell causes a depolarization of the presynaptic terminal 2. This causes the calcium to enter the presynaptic terminal, which causes the release of a neurotransmitter into the synaptic cleft 3. The neurotransmitter will diffuse over the cleft, where it combines with receptors on postsynaptic cell membrane. Here it causes a change in membrane permeability to ions, and changes it's membrane potential 4. Inhibitory neurotransmitters hyperpolarize the postsynaptic membrane. Excitatory neurotransmitters depolarize the postsynaptic membrane |
|
|
|
What is the neurotransmitter released in a presynaptic terminal and what is the receptor of the postsynaptic membrane in a neuromuscular junction?
|
The neurotransmitter is ACh Postsynaptic membrane contains a nicotinic receptor |
|
|
|
What inactivates the ACh neurotransmitter?
|
Acetylcholinesterase will degraded ACh to acetyl Coa and choline on the muscle end plate |
|
|
|
What are the types of synaptic transmissions? |
One to one synapse - Found in neuromuscular junction Many to one synapses - Found in spinal motoneurons - A singel potential in a presynaptic cell is not sufficient to produce a action potential in the postsynaptic cell. Many cells synapse on the postsynaptic cell for it to reach depolarize to the threshold |
|
|
|
ESPS or excitatory postsynaptic potentials What are some excitatory neurotransmitters? |
Inputs which depolarize postsynaptic cells
- Close to threshold, and firing AP Opens channels of K+ and Na+, which depolarizes the membrane potential Excitatory neurotransmitters -ACh, norepinephrine, epinephrine, dopamine, glutamat, serotonin |
|
|
|
IPSP or inhibitory postsynaptic potential What are some inhibitory neurotransmitters? |
Inputs which hyperpolarize the postsynaptic cell, moving it further away from treshold and firing a action potential done by opening Cl- channels, which means that the membrane potential can move towards -90 Inhibitory neurotransmitters are GABA and glycine |
|
|
|
What is the normal range for sodium, potassium, chloride? |
Sodium: 135-145 mmol/L Potassium: 3.5-5-3 mmol/L Chloride: 98-108mmol/L |
|
|
|
What is the normal osmolarity and glucose range? |
Osmolarity: 275-300 mOsm/L Glucose: 70-115 mg/dl |
|
|
|
What is the normal range for Bicarbonate and hydrogen ions? |
Bicarbonate: 22-29 mmol/L Hydrogen ions: 30-50 nmol/L |
|
|
|
What is the normal range for proteins and albumin? |
Protein: 6-8 g/dl Albumin: 3.5-5.5 g/dl |
|
|
|
Monro-Kellie principle |
Volume of brain + volume of blood + volume of cerebral spinal fluid volume is constant Always takes up 15% of CO - 20% of O2 |
|
|
|
What are the slowest and what are the fastest conduction velocities? |
SA node is slowest - 0,01-0,05 m/s Purkinje fibers are fastest - 4m/s |
|
|
|
What is the length of action potential in SA and Av nodes vs working muscle cells? |
SA and AV - 100ms Working muscle: 200-250 ms |
|
|
|
Rapid voltage-dependent Na+ channels |
Has two gates, which both must be open for the Na channels to be opened. Activation "m" gate - Opening during depolarization, closes during plateu phase Inactivation "h" gate - Opened during resting potential - Closes during depolarization, and closed at peak - Closed until repolarization The only time when both gates are open is during the depolarization, and at this stage we have a positive Na+ flow |
|
|
|
Voltage dependent K+ channels |
Only has activation gate
This means when the activation gate is open, the flow of potassium will be positive. Gate is closed during resting potential Opens during depolarization, and stays open until repolarization Will therefore be a positive potassium flow during depolarization, peak of ap, plataue phase and repolarization |
|
|
|
What is the length of the PR, QRS, QT? |
PR: 120-200 ms QRS: 80-100 ms QT: 360-420 ms |
|
|
|
Electric and mechanical systole? |
Electic lasts from E point, just before QRS and until after T wave - Same as QT - 360-420 ms Mechanical lasts from after QRS at J point, until after T wave - 280-320 ms |
|
|
|
Electric and mechanical diastole |
Electrical from T wave until QRS wave - Last 380-440 ms Mechanical from after T wave until J point after QRS - 460-520 ms |
|
|
|
Systolic pressure 1. Left ventricle 2. Aorta 3. LA 4. RV 5. pulmonary arteries 6. RA |
1. 120-140 Hgmm 2. 120- 140 Hgmm 3. 15 Hgmm 4. 20-24 Hgmm 5. 20-24 Hgmm 6. 10 Hgmm |
|
|
|
Diastol pressure 1. Left ventricle2. Aorta3. LA4. RV5. pulmonary arteries6. RA |
1. 5-10 Hgmm 2. 60-80 Hgmm 3.5 Hgmm 4. 1-4 Hgmm 5. 9-15 Hgmm 6. 3 Hgmm |
|
|
|
What are the humoral regulatory mechanisms of the cardiovascular system? |
Adrenal medulla - Secretes epinephrine Hypothalamus - Vasopressin or ADH Kidney and juxtaglomerular apperatus - Renin |
|
|
|
What are local regulatory mechanisms of the cardiovascular system?
|
Bayliss effect Temperature Metabolits Vasoactive substances |
|
|
|
What is the effect of M2, M3 and M5 muscarinic receptors? |
M2 - Negative heart effects M3 - Vasodilation of blood vessels M4 - Vasodilation of cerebral arteries |
|
|
|
Effects on circulation due to standing up? - Primary changes - Compensation - Final result |
Primary changes - Gravity -> blood flows to extremities - Increase in CVP - Decrease in EDV, SV, CO, MAP and blood supply to organs Compensation - Increased HR - Reflex causes vasoconstriction, which increases TPR Final result - CO is normalizing at a lower level than lying - MAP is normalizing |
|
|
|
Effects on circulation due to sitting or lying down? - Primary changes - Compensation - Final result |
Primary changes - Arteric blood pressure decreases in lower part of body - More blood in upper part - Increased CVP, EDV, SV, MAP and blood flow to organs Compensation - Decreased HR - Vasodilation -> decreased TPR Final result - CO is normalizing, higher than standing - MAP is normalizing |
|
|
|
What vessels have the largest pressure and flow rate? |
Arteries of systemic circulation |
|
|
|
What vessels have the largest total cross-section, and smallest diameter and flowrate? |
Capillaries |
|
|
|
What vessels has the highest diameter and capacity of volume, but the smallest pressure and resistance? |
Veins |
|
|
|
What vessels have the highest TPR? |
50-55% of TPR |
|
|
|
Fick principle? |
Use oxygen consumption to determine cardiac output. CO= Oxygen consumption/ (O2 pressure in pulmonary vein - O2 pressure in pulmonary artery) |
|
|
|
What is the heart index? |
Heart index= CO/BSA m2 BSA is body surface area |
|
|
|
Whats is does poesieuilles equation say that viscosity, radius and length affect the resistance? |
Resistance is directly proportional to viscosity. As it increases, the resistance will increase. Resistance is directly proportional to the length of the vessel The resistance is inversely proportional to the fourth power of the vessel radius - if the blood vessel radius decreases by a factor of 2, it will increase the resistance by a factor of 16(2^4) |
|
|
|
What is reynolds number? |
Reynolds number predicts whether or not the blood flow will be turbulent Greater chance when reynolds number is increased |
|
|
|
What is vasomotion? |
Spontaneous oscillation in tone of blood vessel Independent from heart beat, innervation and respiration |
|
|
|
What other effects than vasodilation does NO have? |
Antagnoise the effect of AII, ET-1, and NA Inhibit aggregation of thrombocytes Inhibit adhesion of leukocytes Inhibit proliferation of sm cells Anti-inflammatoric effect |
|
|
|
boosts venous return - Vis a tergo |
The work of the heart pressure gradient from the left ventricle to the right atrium |
|
|
|
boosts venous return - Vis a fronte |
Suckling effect on large veins due to contractions of heart muscle Also negative thoracic pressure during inspiration |
|
|
|
boosts venous return - Vis a laterale |
Muscle pump Sympathetic tone Thoracoabdominal pump Arterial pulsation |
|
|
|
Thoracoabdominal pump |
Inspiration -Decreased intrathoracic pressure, increases the venous return Expiration - Increased intrathoracic pressure, decreases the venous return |
|
|
|
Definition of tachypnea, hyperpnea, bradypnoe, hypohnoe, apnea, apneusis, dyspnea and orthopnea? |
Tachypnea - increased respiratory frequency Hyperpnea - increased respiratory minute volume Bradypnoe - Decreased respiratory frequency Hypopnoe - Decreased respiratory minute volume Apnea - Breathing pause at expiration Apneusis - Deep, gasping inspiration with a pause at full inspiration Dyspnea - Shortness of breath Orthopnea - Shortness of breath, referring to heart disorders |
|
|
|
What parameters can be measured with a spirometry? |
Resting respiration - static parameters - Volume and capacities Tidal volume, inspiratory volume, expiratory volume, vital capacity, inspiration capacity The residual volume can not be measured- Because of this TLC and FRC can not be measured During heavy breathing - dynamic parameters - Velocities, volume/time curves Velocities - Peak expiration flow - Forced expiratory flow Dynamic volumes - FEV *Volume of air which can forcibly be blown out in a unit of time after full inspiration - Tiffeneau-index: FEV1/FCV propotion *Normally above 70% *Decreases due to big flow resistance -> airway obstruction - FIV *Forced inspiratory volume, volume which can forcible be inspirited in one unit of time *Decreased caused by upper airway obstruction |
|
|
|
What causes obstructive respiratory disorders? |
Limitation of airflow Obstruction of the upper airways Increased resistance of the lower airways Decreased elastic resistance |
|
|
|
What causes restrictive disorders? |
Decreasing of ventilation surface or expansibility (Belt) |
|
|
|
What is the site of action for aldosterone in the kidney and what is the effect? |
Collecting tubule and duct Increased NaCl and H2O reabsorption and increased K+ secretion |
|
|
|
What is the site of action for Angiotensin II in the kidney and what is the effect? |
Proximal, thick ascending, loop of Henle, distal tubule and collecting tubule Function is increased NaCl and H2O reabsorption, and increased H+ secretion |
|
|
|
What is the site of action for antidiuretic hormone or ADH in the kidney and what is the effect? |
Distal tubule, collecting tubule and duct H2O reabsorption |
|
|
|
What is the site of action for Atrial natriuretic peptide or ANP in the kidney and what is the effect? |
Distal tubule, collecting tubule and duct Decreased NaCl reabsorption |
|
|
|
What is the site of action for parathyroid hormone in the kidney and what is the effect? |
Proximal tubule, thick ascending loop of Henle´s and distal tubule Decreased PO4 reabsorption, Increased Ca++ reabsorption |
|
|
|
Tritiated water |
A marker for total body water Distributes to wherever water is found |
|
|
|
Mannitol |
A marker for ECF Mannitol is a large molecule, which can't cross cell membranes, and is therefore excluded from the ICF |
|
|
|
Evans blue |
Marker for plasma volume the dye will bind to serum albumin and therefore is confined to the plasma compartment |
|
|
|
What substances can be used to measure total body water? |
Tritiated water, D2O, antipyrene |
|
|
|
What substances can be used to measure ECF?
|
Sulfate, inulin, mannitol |
|
|
|
What substances can be used to measure plasma? |
RISA and evans blue |
|
|
|
What substances can be used to measure interstitial? |
none, must be measured indirectly ECF - plasma volume |
|
|
|
What substances can be used to measure ICF |
measured indirectly TBW-ECF volume |
|

