![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
108 Cards in this Set
- Front
- Back
Anabolic
|
need energy
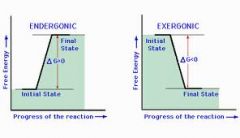
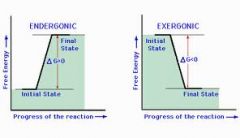
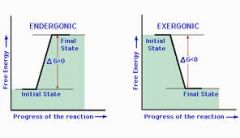
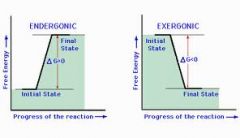
endegonic(+delta G), Delta S decreases, for growth, repair synthesize starch, glycogen) |
|
Catabolic
|
releases free Energy
exegonic(-delta G), Delta S increases, provides bio-synthesis, precursor for Anaerobic,Aerobic hydrolysis of macromolecules or biological oxidation |
|
|
Anaerobic/Aerobic
|
based on oxygen
Mitochondria is oxygen dependent |
|
|
linkage of Anabolism, Catabolism through ATP
|
linkage through prod. of ATP. ATP is used in various stages in anabolism.(carbs, fats, proteins)
ATP/ADP pairing linked |
|
|
catabolism energy yielding
|
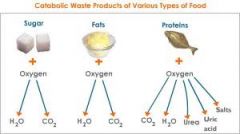
Energy yielding metabolism,utilizes energy, provides heat, gives out metabolic products
|
|
|
universal energy coupler
removal of terminal phosphate |
term. phosphate can be removed fairly easy>b.c. of neg charge on the phosphate group. >lots of free -e are delocalized and arrange themselves> delocalized >lowest energy state.>resonance stabilization.
|
|
|
3rd phosphate -7.3kcal
|
3rd phosphate undergoes hydrolysis ATP to form ADP> highly exergonic>>(delta G)-7.3 kcal
|
|
|
phosphate and its electron delocalization
|
`highly exergonic because of charge repulsion- each phosphate has a neg. charge because of pH of the cell.
resonance stabilization.> electrons delocalized over bond> lowest energy state>electrons are deloc. and rearrange themselves |
|
|
ATP/ADP Position donor acceptor
|
occupies an intermediate position, therefore it can serve as a phosphate donor or acceptor.ATP donates phosphates with less free energy.ADP accepts phosphate from those of higher free energy>>>-7.3 is middle
|
|
|
exergonic transfer of phosphate groups
|
has lots of free energy
PEP(pyruvate) transfers its phosphate group exergonicaly onto ADP to form ATP,and ATP can phosphorylate glucose Exergonicaly but reverse reaction is not possible endproduct is G6P |
|
|
ATP /ADP transfer and release of energy within a cell
|
( reversible means of conserving), transferring and releasing energy within the cell. As catabolic processes in the cell the energy released is coupled to ATP/ADP system such as the free energy drives the formation of ATP.ATP is used to do work
|
|
|
ATP creation during oxidative catabolic state
|
ATP is generated during the oxidative catabolism of nutrients and is used to do cellular work
|
|
|
Production of ATP during anaerobic state and end result of yeast , humans...
|
other organisms (bacteria): prod. ethanol. Co2 =end product
in higher organisms > lactate is the end product + heavy exercise(humans) generates modest ATP to do work |
|
|
In Aerobic condition /creation of ATP
|
presence of oxygen, ATP is 20 x higher per glucose molecule, nutrients are catabolized to Co2 and H2O, releases heat in liver
|
|
|
oxidation reduction
oxidation is loss ,reduction is gain... |
of electrons and protons
all oxidizable compounds that undergo highly exergonic reactions |
|
|
reduction
|
addition of electrons / protons in the endergonic process. hydrogenation.(to bond with hydrogen)
|
|
|
oxidation
|
dehydrogenation/exergonic
electrons removed/protons liberated |
|
|
electron acceptors
|
final electron acceptor , oxygen-
via intermediates as NAD+(coenzyme)>they function with enzymes as electron carriers or small function groups NAD+------>NADH + H+ |
|
|
cofactors in org. minerals...
|
assist in enzyme function(Mg,Iron)
act as electron carriers. |
|
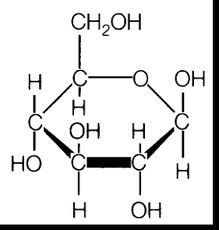
glucose
|
most important oxidizable substrate in metabolism,
main blood sugar comes from plentiful dietary sources one half of the plant ,disaccharide , sucrose(source) glucose is an aldohexose> a 6 carbon sugar with a terminal carbonyl group. |
|
|
alpha D glucose
|
the repeating unit of starch and glycogen .hydroxyl group points downwards
|
|
|
Beta D glucose
|
the repeating unit of cellulose
hydroxyl group points upwards |
|
|
glycolysis. production of ATP molecule
|
Anaerobic
most energy without oxygen highly exergonic= -686kcal not in mitochondria, but in cytoplasm -delta G |
|
|
Glycolysis Pyruvic acid
|
glucose is broken down into two mol. of pyruvic acid>occurs in the cytoplasm of animal cells plant cells, org. 6 enzymes work in the metabolic pathways. 6 carbon molecule is split into two 3 carbon molecules>each,part. oxid.to generate 2 ATP molecules per glucose
|
|
|
1 and 3 step of glycolysis
|
ATP energizes molecules, 2 ATP mol. are expended. 6 carbon molecule splits into two 3 carbon comp.-->and form pyruvic acid
|
|
|
glycolysis latter stage
|
4 ATP molec. are synth.2 ATP{ molc. are used.net gain 2 ATP.
Another reaction of glycolysis->NAD > to NADH NADH coenzyme will later be used |
|
|
during glycolysis how many NADH are produced
|
2
|
|
|
glycolysis is inefficient because
|
because much of cell energy remains in 2 molecules of pyruvic acid
does not use any oxygen,> anaerobic> for bacteria and fermentation yeast> glycolysis is the only source of energy |
|
|
overview of glycolysis
phase 1 preparation and cleavage |
glucose + 2 ATP = 2 G3P + 2 ADP
|
|
|
phase 2 oxidation and ATP generation
|
G3P + NAD (+) +ADP+ P>NADH (+) + ATP
|
|
|
PHase 3
|
Pyruvate formation and ATP generation: = Pyruvate + ATP
4 produced , 2 used |
|
|
overall reaction from 1 to 10
|
=>2 Pyruvate + 2 ATP
net prod. is 2 mol. of ATP+2 mol. of pyruvate!! |
|
|
from glycolysis(pyruvate) in Aerobic condition..
|
O2 present, Pyruvate is conv. to Acetyl CoA.
here the pyruvate is Oxidized(NAD =>NADH) looses 1 carbon which turns to co2 and leaves the cell |
|
|
Anaerobe condition
|
no O2 , pyruvate is red. so that (NADH can be oxidized to.NAD) Lactate(animals)
ethanol and Co2 in (Plant cells and yeasts) |
|
|
glycolysis vs. glycogenesis
|
reciprocal regulation via allosteric activation and inhibition
|
|
|
AMP
|
Activates glycolysis but inhibits glyconeogenesis (allosteric regulation)
|
|
|
when (ATP)is low and AMP is high,..
|
then the cell is on low energy>AMP activates glycolysis and inhibits Gluconeogenesis
|
|
|
Gluconeogenesis
|
is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids.
|
|
|
If ATP is high and and AMP decreases
|
than the glycolysis is inhibited and Gluconeogenesis is activated.
AMP will activate glycolysis to produce ATP>dependent on ratio of ATP |
|
|
cori cycle
|
link between glycolysis in muscle cells and Gluconeogenesis in the liver.
|
|
|
glycolysis
|
) is the metabolic pathway that converts glucose into pyruvate, The free energy released in this process is used to form the high-energy compounds ATP and NADH
|
|
|
Cori cycle
|
muscle get ATP from glycolysis,in Anaerobic period.
lactate is transported by blood to liver>reoxidized to pyruvate. Pyruvate is substrate for gluconeogenesis in liver.>generates glucose and >back to blood |
|
|
Aerobic respiration
|
cell. respiration , flow of electrons through or within a membrane,from reduced coenzyme to an electron acceptor, usually generates ATP
|
|
|
ELectron acceptors
|
NADH, FADH, Coenzyme Q(ubiquinone) are aerobic
Anaerobes are S (sulfur)/H2s(hydrogen sulfide), H+/H2, Fe3/fe2 |
|
|
role of mitochondria in cellular respiration
|
oxidation of glucose & other sugars begins in cytosol with glycolysis producing pyruvate
|
|
|
pyruvate transported across membrane...
|
across inner mitoch. memb. and is oxydized within the matrix to acetyl CoA.>primary substrate for TCA cycle. Acetyl CoA can also be formed by B oxidation of fatty acids.
|
|
|
electron transport in mitochondria
|
is is coupled to proton pumping , with the energy of electron transport conserved as an electrochemical proton gradient across the membrane of the mitochondria.
|
|
|
Synthesis of ATP from ADP +P
|
the energy of the proton gradient is used to drive the synthesis.
|
|
|
Electron transport chain
Oxygen |
terminal electron acceptor(O2)
allows for continues redoxidation of NADH and other reduced Coenzymes. |
|
|
coenzymes accept ...
|
electrons during the stepwise oxidation of organic intermediates
derived from pyruvate. electron are then transferred to O2 via membrane bound electron carriers> indirectly generating ATP |
|
|
regulation of TCA cycle
|
allosterically regulated.
All 4 NADH generating enzymes are inhibited by NADH Allosteric regulation occurs via NADH, ATP, and acetyl COA ar various points. High AMP=High PDH High ATP=Low PDH High ADP =High Isocitrate High NADH= Low isocitrate |
|
|
summery of the TCA cycle
|
Glucose produces 4 ATP >2 from glycolysis and 2 from TCA
10 NADH that give@3 ATPs= 30 ATP 2 FADH taht give 4 ATPs and byproduct of 6 CO2 |
|
|
overview: TCA cycle
|
Acetate enters TCA cycle as acetyl CoA and is joined to a 4 carbon acceptor(oxaloacetate) to form citrate>a 6 carbon molecule
|
|
|
decarboxylation of TCA
|
occurs at 2 steps in the cycle so that the input of two carbons is balanced by the loss of two carbons as CO2
|
|
|
TCA cycle oxidation occurs where?
|
at 4 steps, with NAD+ as the electron acceptor in 3 reactions and FAD as electron acceptor in one case
|
|
|
TCA cycle ATP generation
|
ATP generated at 1 point with GTP as the intermediate (in animal cells)
|
|
|
TCA cycle oxaloacetate
|
one turn of cycle is completed upon regeneration of ocxaloacetate, the original 4 carbon acceptor
|
|
|
MITOCHONDRIA
outer membrane |
phospholipid synthesis
fatty acid desaturation fatty acid elongation |
|
|
Inner membrane
|
electron transport
Oxidative phosphorilation metabolite transport |
|
|
Matrix
|
Pyruvate oxidation
TCA cycle Beta oxidation of fats DNA replication RNA synthesis (transcription)protein synthesis |
|
|
mitochondria and the pyruvate
|
Pyruvate has to cross the outer membrane to get into mitoch.
Electron transport chain in inner membrane of the cristae> Matrix side of TCA cycle> cant happen without pyruvate oxidation |
|
|
F1 and F0 complex
|
Each F0 and F1 complex is attached together by a protein stalk, they constitute a functional ATP synthase
|
|
|
elctron transport chain
|
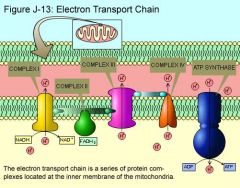
|
|
|
electron transport chain H+ gradient
|
H+ is pumped into intermediate membrane space>ATP is produced in membrane
inside membrane: hydrophobic transport electron through membrane allows for H+ gradient>ATP synthesis |
|
|
number of electron carriers
|
1 flavoprotien
2 iron sulfur protein 3 cytochromes 4 copper containing cytocranes5 5 co enzyme Q (quinone) 2 3 4 contain a prosthetic group |
|
|
Iron sulfur protein
|
deals with FADH2
|
|
|
cyanide
|
stops electron transfer
|
|
|
electron transport chain summary cristae
|
-occurs in cristae
-in cristae: cytochromes and coenzymes >act as carrier molecules and transfer molecules. these accept high energy electrons and pass it along energy of electrons transports protons across membrane in to outer compartment of mitoch. |
|
|
electron transport chain overall
|
prod. of cellular respiration
h2o, CO2 , 34 ATP from each molecule of glucose +4 atp= 38 |
|
|
chemiosmosis H gradient
|
ref to movem. of protein across the membrane to generate ATP
pumping protons from the inner to the outer compartment(memb. of mito.) A gradient is est.H+ go down the gradient> generate ATP using ADP + P. |
|
|
Chemiosmosis
|
38 molecule ATP> cannot be stroed for long time
cellular respiration must continue to regenrate ATP.Each ATP releases 7.3 Kcal of energie |
|
|
intracellular components
|
many metabolic processes in cells
involves in bio synthesis and trafficking must be tightly regulated> so each received necessary components |
|
|
rough ER
smooth ER |
protein synthesis, processing and sorting
detox, prod. of lipids.phospholipids, steroids, hydrolysis of glycogen |
|
|
endosomes
|
sorting of materials entering the cell by endocytosis from lysosomes
|
|
|
Lysosomes
|
digestion of unwanted material
|
|
|
Peroxisomes
|
house peroxide generating reactions essential role in fatty acid oxidation
bio synthesis of lipid membranes |
|
|
endomembrane system consist of
|
rough and smooth ER
Golgi apparatus endosomes lysosomes nuclear envelope allows for continuous transport of materials through the cell |
|
|
intracellular compartments
|
rough ER is connected to nucleus
|
|
|
rough ER
Flattened SAC |
biosyn. and processing of protein-membrane bound
Physiological Sac: Ribosomes face from lumen.Contain rRNA |
|
|
rough ER
|
contain transitional elements-transition vesicles shuttle lipids and proteins to golgi complex
|
|
|
rough ER in organelle structure and membranes
|
used for organelle structure & function,(for enzyme) plasma membrane) some exported out of cell
adds carbohydrate groups to proteins-glycolysilation recognition and removal of misfolded polypeptides. (ERAD) |
|
|
smooth ER
drug tetox |
P450enzyme (in liver)adds a hydroxyl (OH) group to a large, hydrophobic molecule.
hydroxylated drugs eliminated cause they are more water soluble |
|
|
smooth ER carcinogens p450
|
different P450 adds OH to polycyclic molecule using aryl hydrocarbon hydoxylase (AHH) converts pot. carcinogens to active forms
smoking increases activity of AHH |
|
|
SMOOTH ER
carbohydrate metabolism |
removes a phosphate from glucose
dephosphorilization allows glucose to leave cell via channel protein (permease) G6p high in liver, kidney and intestinal cells,low in brain and muscle>L: they need G6p for their own need |
|
|
Smooth ER
and its calcium storage |
calcium storage >pumps CA++ to SR
membrane produc. > most phosphor lipids derived from smooth ER > req. flipase enzyme to export across the ER memb.into cytosol. |
|
|
golgi complex
|
loc. near ER (CGN=cis golgi network) assc. with nucleus
TGN (trans ) faces away from the ER |
|
|
transport through golgi complex
stationary |
each compartment is stable structure shuttle vesiceles bud from one cisterna and fuse with next
cis to trans |
|
|
cisternal maturation
|
cisternae are transient complexes that alternante between CGN and TGN from cis to trans> froms and releases vesicle
|
|
|
protein glycolysation
glucan synthatase |
proteins become glycosilated
2 main enzymes :glycon synthasase=catalize formation of oglisaccarides |
|
|
glycosyl transfease
|
attach carbohydrate group to protein
|
|
|
protein trafficking
|
constitutive secretion: prod. of mucus, trachea, ongoing process>does not regulated secretion does req.external stimulus. ( neurotransmitters, hormones)
|
|
|
exocytosis
|
polarized secretion, the process is dependent on CA++
thought to activate protein kinase signal is usually a neuro transmitter hormones NT activate second messengers |
|
|
endocytosys
|
invaginated vesicle develops into early endosome
from plasma membrane back into the cell >retro>transport enzyme is precursor to lysosomes |
|
|
phagocytosis
|
removes toxic particles , include neutrophils and macrophages,bind to surface
synthesize toxic levels of h2o2 |
|
|
receptor mediated endocytosys
|
also called clathyndependent endocytosis
ingests hormones and enzymes,growth factors ,LDL, receptor mediated uncoated vesicle fuses with TGN to form early endosome>developes to lysosome |
|
|
receptor mediated endocytosis
|
hetrophage lysosome out side of cell
auto phage from inside the cellclathryn coated pits. |
|
|
formation of lysosomes
|
involved in nutrition , defense, recycling differentiation
|
|
|
central nervous system
|
brain and spinal cord
|
|
|
peripheral nervous system
|
sympatic parasympatic enteric
|
|
|
autonomic nervous system
|
invouluntary
|
|
|
somatic nervous system voluntary
|
motor pathways automatic and somatic
|
|
|
structure of nerv cell
dendrite |
contain receptors > neurotransmitters
> has nucleus Soma>leads to axon |
|
|
axon (generating and conducting region)
|
axon hillick >generates impulse nerve of Ranvier
direction from axon to axonal terminal solitary conduction electronic conduction |
|
|
membrane poteintial
|
inside neg. resting potentail > membrane potential
-70 m volts is negative to respect of outside |
|
|
resting membrane potential
|
if neuron is not sending a signal >its at rest
inside is neg. to outside. K+ can cross easy CL- and NA+ have more difficult time neg. charged protein molecules inside the neurone cannot cross the membrane |
|
|
resting membrane potential
|
contain NA+ K+ pumps
uses 3 ATP to simultaneously pump 3 sodium ions out of the cell and 2 K+ in selective permeable NA/K ATP ase pump restores the membrane potential |
|
|
resting membrane potential
|
at rest membrane allows to leakage down the gradient
|
|
|
resting membrane potential
overall |
K+ passes easy
CL- and NA+ have difficulties neg . charged protein mol. (A-) cannot pass membrane NA+K+ATPase moves 3 NA+ out for 2 K+> imbalabce called resting membrane potential |

