![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back

A physical property is an aspect of matter that can be observed or measured WITHOUT CHANGING IT.
|

Examples are: color, size and shape
|
|
|
Matter occurs in what three physical forms, called states?
|

Solid
Liquid Gas |
|
|
Solubility:
|

Ability to dissolve in a liquid.
(If you mix sugar in a glass of tea, it remains sugar.) |
|
|
Ductility
|

How easy it is for something to be pulled into a wire.
|
|
|
Malleability
|

The ability to be pounded into thin sheets.
Example: Aluminum Foil has high malleability. |
|
|
Thermal Conductivity
|

is the rate at which a substance transfers heat (thermal) energy
If something does not transfer heat energy well, it is a poor conductor. Poor Conductors are good insulators |
|
|
Physical Change -
does not produce a new substance. |

If I crush a soda can is it still a soda can? Yes. Only the size changes.
|
|

If you break a piece of chalk, is it still chalk?
|

Yes. Only the size of the chalk changes.
Physical Change Examples: Freezing water to Ice Cutting your Hair Mixing Oil and Vinegar |
|
|
Chemical Properties are ONLY SEEN during or after a chemical change
|

Tree is on fire, this is an example of the property of flammability.
|
|
|
Chemical Change - (REACTION)
always produces a new substance |

Occurs when a substance (or substances) is converted into a different substance (or substances).
Remember Different substances before and after. |
|
|
Density
|
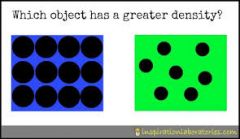
A measure of mass per unit of volume. The more tightly packed the molecules of an object, liquid or gas are, the more dense we say they are.
Ex. box on left has more density. |

