![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Clostridial fermentation calculations |
1. How many H2 from NADH reox? (rest are from Fd reox) 2. Will use 2 NADH per Glu. 3. 2*(Glu)-(Number reox)= NADH left to reox 4. Must make 1 butyrate for every 2 NADH reox. 5. Will use 2x as many Acetyl-CoA (out of 2x glucose) to make but. 6. Rest of acetyl CoA will make 1x acetate 7. ATP: 2x glucose (EMP), 1x but, 1x acetate |
|
|
Reactions that make NADH and FDred |
C: pyruvate + CoA + FDox ->CO2 + acetylCoA + FDred
P: pyruvate + CoA + NAD+ ->CO2 + acetylCoA + NADH + H+
Both: glyceraldehyde3P + NAD+ -> 1,3bisPglycerate + NADH + H+ |
|
|
Reactions that make NAD+ and FDox |
C: crotonyl-CoA + NADH + H+ -> butyryl-CoA + NAD+
acetoacetyl-CoA + NADH + H+ -> 3-hydroxybutyryl-CoA + NAD+
P: oxaloacetate + NADH + H+ -> malate + NAD+
fumarate + NADH + H+ -> succinate + NAD+ |
|
|
Reactions that make ATP |
C: butyrylP + ADP -> butyrate + ATP
Both: phosphoenolpyruvate + ADP -> pyruvate + ATP
1,3bisPglycerate + ADP -> 3Pglycerate + ATP
acetylP + ADP -> acetate + ATP |
|
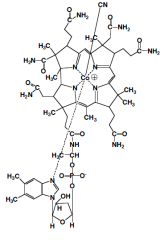
|
5' deoxyadenosyl-cobalamin (B12)
Fxn: H rearrangement
forms a free radical used to remove H from substrate |
|
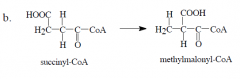
What cofactor is involved in this reaction? |
5'-deoxyadenosyl-cobalamin (B12) |
|
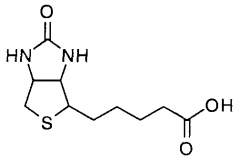
|
biotin Fxn: transcarboxylation, carboxylation/decarboxylation |
|
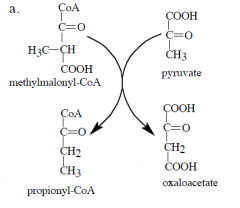
What cofactor is involved in this reaction? |
biotin |
|
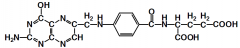
|
5,6,7,8-tetrahydrofolate (folic acid/para-aminobenzoic acid) Fxn: one carbon transfer (NOT carboxy transfer) used by acetogens to form methyl group of acetate |
|
|
function of ferredoxin |
electron transfer |
|
|
enzymes used to metabolize pyruvate |
pyruvate:ferredoxin oxoreductase
pyruvate formate lyase (enterics) |
|
|
metals besides Fe used by hydrogenases |
Ni, Se |
|
|
why is ATP needed to fix N2 |
to lower redox potential of electrons transferred to nitrogenase |
|
|
how do pyruvate-formate lyase and methylmalonyl-CoA mutase cleave C-C bonds |
free radical |
|
|
what mods to iron in Fe-Fe hydrogenases allow them to make H2 |
CO or CN bound to Fe |
|
|
main functions of methanogens |
hydrogen and acetate use |
|
|
why is T. maritima's hydrogenase able to produce H2 from NADH AND FDred (a lot of H2) |
coupled reactions; production of H2 from FD is favorable, used to drive H2 production from NADH which is unfavorable |
|
|
why is it difficult to make 12 H2 from glucose |
reactions that take hydrogen from acetate, butyrate, propionate (intermediates in anaerobic degradation of glucose) are endergonic (unfavorable thermodynamically) |
|
|
fxn of MoFe protein in N2 fixation |
reduces N2 to 2 ammonia (NH3) |
|
|
would G- bact that fermented glucose and made 2,3-butanediol be a health risk? |
no, 2,3-butanediol indicates bact from plants not fecal matter |
|
|
why is colon fermentation good for your health/ why would switching from red meat to grains help colitis |
butyrate is important for epithelial cell devop., colon cancer/colitis, immune system control. enterics will turn meat into toxic H2S in small intestine, but grains/veggies have nonreactive starch that will make it to large intestine and get fermented to acetate, propionate, butyrate |
|
|
why would a digestor run at fast retention times cause acetate, propionate, and butyrate to accumulate
why would BESA cause this |
too fast for acetate-using methanogens and syntrophic metabolizers to grow. H2-using methanogens grow faster so high H2 pp is not problem
methanogens are inhibited, usually keep H2 low so others can make acetate, ATP, etc, but without low H2 these reactions are therm unfavorable so use the acid producing pathway |
|
|
function of Fe protein in N2 fixation |
electron acceptor for FD, transfers to MoFe. uses ATP to lower redox potential of electrons for transfer. |
|
|
how do acetogens reoxidize NADH |
use CO2 as an electron sink
|
|
|
major metabolic groups in cow rumen |
methanogens, cellulose-fermenting, syntrophic |
|
|
why do sulfate reducers use adenosine 5' phosphosulfate intermediate instead of doing it directly |
would require too much energy to lower redox potential of the electrons, so use ATP this way |
|
|
what enzyme would you think you need to get H2 from Glu that most anaerobes don't have |
bifurcating hydrogenase or pyruvate:ferredoxin oxidoreductase
want to form H2 and regenerate electron carriers, could increase ATP yield |
|
|
if a propionic doesn't have a functional PEP carboxytransphosphorylase, what would you need to add to the medium to allow growth? |
malate, succinate, or fumarate; can go to methylmalonylCoA and restart cycle back to oxaloacetate. without this enzyme it couldn't make PEP into oxaloacetate using CO2 fixation so it has to use the other pathway. |
|
|
pyruvate:ferredoxin oxidoreductase
word eq, cofactors, org/condition |
pyruvate + CoA + FDox -> acetylCoA + CO2 + FDred + 2 H+
thiamine pyrophosphate, CoA, FD
Clostridia, acetogens anaerobic metabolism to get pyruvate to acetylCoA without producing any NADH, use CO2 and make carbon in high PP of H2 |
|
|
pyruvate:formate lyase
word eq, cofactors, org/condition |
pyruvate + CoA -> acetylCoA + formate
free radical carriers like glycine, tyrosine
in enterics, free radical cleavage, in mixed acid fermentation |
|
|
alpha-acetolactate synthase
word eq, cofactors, org/condition |
2 pyruvate -> CO2 + alpha-acetolactate
thiamine pyrophosphate
enterics using 2,3-butanediol fermentation |
|
|
molar growth yield to ATP production |
(X gram cells per mol Glucose (given))/(10.5g cell/ mol ATP) = X ATP/Glu |
|
|
how would you do an experiment to see if an org uses ED or EMP pathway |
label 1 carbon: ED will give labeled CO2, EMP won't
label 3 carbon: EMP will give labeled CO2, ED won't
|
|
|
randomizing v. acrylate pathway for propionate metabolism |
acrylate: one product with one labeled carbon
randomizing (methylmalonylCoA): two products with two different labeled carbons, since intermediate is symmetric |
|
|
how do clostridia reoxidize FD? |
by using hydrogenase to make H2 |
|
|
why would pyruvate:ferredoxin oxidoreductase be selected for in obligate anaerobes? |
makes hydrogen production favorable even at high PP |
|
|
phosphoenol pyruvate-carboxyltransphosphorylase equation |
diphosphate + oxaloacetate -> phosphoenolpyruvate + CO2
|
|
|
function of P clusters in nitrogenase |
electron transfer (2) so nitrogen can go to ammonia |
|
|
function of H cluster in Fe-Fe hydrogenase |
active site, where catalysis of H2 production happens |
|
|
what can you conclude about an org that has Co dehydrogenase/acetyl-CoA synthase |
it is anaerobic and fixes CO2 |

