![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
|
How are lipids transported in the blood?
|
as macromolecular particles called Lipoproteins
*the main blood lipids cholesterol & triglycerie have to be packaged into lipoprotein balls for transport in blood or they would just float to the top like fat in chicken soup |
|
|
Metabolic disorder with increased levels of Lipoproteins
|
Hyperlipidemia
|
|
|
Term that refers to an increased plasma triglyceride level
|
Hyperlipemia
|
|
|
List the names of the Lipoproteins that transfer lipids into arterial walls
|
1. LDL
2. IDL = intermediate-density 3. VLDL = very low-density 4. Lipoprotein (a) = Lp(a) |
|
|
Describe the structure of Lipoproteins
|
Lipid membranes = contain free cholesterol & phospholipids arranged in a monolayer
Hydrophobic core: -Cholesterol esters = cholesterol molecule linked to a fatty acid -Triglycerides = glycerol molecule linked to 3 FA's Apolipoproteins = high-molecular weight protein molecules are integrated into the lipid membrane |
|
|
What Lipoproteins contain Apolipoprotein B100?
|
VLDL
IDL LDL Lp(a) **binds to LDL receptor; mediates VLDL secretion |
|
|
What Lipoproteins have Apolipoprotein B48?
|
Chylomicrons = delivers dietary Triglycerides to peripheral tissues & dietary Cholesterol to the Liver
|
|
|
Lipoproteins that function as lipid production & transport
|
VLDL
IDL LDL |
|
|
Lipoprotein that transfers cholesterol & apolipoproteins among different lipoprotein subclasses & to the liver
|
HDL
|
|
|
List the Lipoprotein from heaviest (more protein) to lightest (more fat)
|
1. HDL
2. IDL 3. LDL 4. VLDL |
|
|
Describe Chylomicron & Fat Absorption
|
1. Cholesterol esters & Triglycerides are hydrolyzed to form Cholesterol & Fatty Acids
2. Cholesterol & Fatty Acids are solubilized by Bile Salts & Phospholipids are secreted into the Small Intestine 3. Solubilized cholesterol & fatty acids are absorbed by enterocytes in the duodenum & proximal jejunum 4. Long-chain FA's in enterocytes form Chylomicrons 5. Chylomicrons secreted into lymph, thoracic duct, & veins 6. Triglycerides from circulating chylomicrons are hydrolyzed by lipoprotein lipase in adipose & muscle tissues 7. Chylomicron remnants are formed & removed by binding ApoE to hepatic LDL receptor |
|
|
What accounts for 20% of the body's cholesterol? 80%?
|
20% = food
80% = hepatic synthesis |
|
|
Describe what happens after a fatty meal in the liver
|
1. Hepatocytes initiate Triglyceride & cholesterol synthesis
2. Triglycerides & cholesterol fused with Apolipoprotein B100 to form mature VLDL that is secreted into blood 3. Lipoprotein Lipase in muscle/adipose tissues remove Triglyceride core of VLDL to form remnants IDL & LDL 4. LDL is removed by liver & macrophages |
|
|
"bad cholesterol" that makes up 65-75% of total plasma cholesterol
|
LDL
|
|
|
What is elevated LDL a major risk for?
|
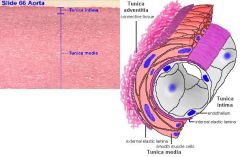
Atherosclerosis
-LDL that is not taken up by hepatic LDL receptors migrates into vascular INTIMA to be taken up by scavenger receptors on phagocytic cells -oxidized LDL accumulates in Foam cells |
|
|
Where is HDL synthesized?
Explain how it draws free cholesterol from cholesterol-rich cells |
Liver & Intestine
1. Cholesterol is esterified by Lecithin:Cholesterol Acyl Transferase (LCAT) 2. forms larger hydrophobic core of Cholesterol Esters in HDL 3. HDL transfers cholesterol esters to VLDL, IDL, LDL & Chylomicrons *HDL decreases the amount of cholesterol available for tissue deposition by removing it from macrophages & promoting its return to the liver |
|
|
What is the current optimal level of LDL?
|
< 100
|
|
|
How are serum lipid levels measured?
|
after 10-hour fast
|
|
|
What is the common ending for the HMG-CoA Reductase Inhibitors?
|
"STATINS"
-Atorvastatin -Fluvastatin -Lovastatin -Pravastatin |
|
|
What is the mechanism of all Statins?
|
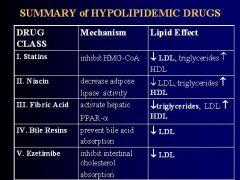
Inhibit HMG-CoA Reductase to:
1. reduce hepatic formation of Cholesterol 2. increase high affinity LDL receptors in liver cells & lower plasma LDL 3. decrease plasma Triglycerides 4. increase HDL |
|
|
Which STATINS are prodrugs?
Which ones are active congeners? |
Lovastatin & Simvastatin
Atorvastatin, Fluvastatin, & Pravastatin = FAP "LS = Latent Statins" |
|
|
GI absorption is almost complete for ______ & varies from 40-75% for the other STATINS; all have high 1st-pass hepatic extraction & mostly excreted in ______
|
Fluvastatin
Bile |
|
|
What does HMG CoA Reductase do?
|
converts HMG CoA -> Mevalonate
-leads to formation of Cholesterol |
|
|
Explain the step-wise mechanism of action of the STATINS
|

1. Competitive inhibition of HMG CoA Reductase which catalyzes Cholesterol synthesis
2. decreased Cholesterol in Hepatocytes causes cleavage of sterol regulatory element binding protein (SREBP), a cytoplasmic transcription factor 3. SREBP diffuses into the nucleus to bind to Sterol Response Elements (SRE) & cause up-regulation of the LDL receptor 4. increased LDL-R results in increased plasma LDL clearance & reduced circulating LDL levels |
|
|
List the 4 Pleiotropic Statin effects
|

-
|
|
|
What is the 1st line therapy for elevated LDL levels?
|
Statins
|
|
|
At what time of day are administration of STATINS most likely to be effective?
What enhances absorption? |
Night when most of cholesterol synthesis occurs
When taken with food |
|
|
What do STATINS significantly reduce the mortality from?
|
MI & Cardiovascular Disease patients
|
|
|
What indicates STATIN toxicity?
|
serum Aminotransaminase = Hepatotoxic = increased LFT's
|
|
|
How is Statin heptatic toxicity manifested?
|
Malaise
Anorexia precipitous fall in LDL *more pronounced in patients with liver disease or alcoholism |
|
|
Which Statins are mainly catabolized by CYP-3A4?
|
Atorvastatin
Lovastatin Simvastatin *SAL - Charles Barkley (3a4) |
|
|
What drugs inhibit CYP-3A4 and can increase the concentration of Atorvastatin, Lovastatin, & Simvastatin?
|
Macrolides = Erythromycin
Cyclosporine Ketoconazole Fibrates Grapefruit juice |
|
|
Which drugs can inhibit CYP-2C9 & increase levels of Fluvastatin & Rosuvastatin
|
Ketoconazole
Metronidazole Sulfinpyrazone Amiodarone Cimetidine MASCK the effects of 2C9 |
|
|
Which Statin is not metabolized by P450 & is preferred whenever concurrent treatment with other drugs is required?
|
Pravastatin
*PRivileged-statin |
|
|
What are the toxicities of the Statins?
|
Hepatotoxicity
Myositis/Rhabdomyolysis = increased Creatine Kinase activity -skeletal muscle pain, tenderness, or weakness |
|
|
What serum tests should be done before treatment & every 6-12 months thereafter when taking STATINS?
|
1. Aminotransferase = hepatotoxicity
2. Creatine Kinase = rhabdomyolysis |
|
|
What is Niacin?
|
Water soluble vitamin (B3) that is converted to the Amide, incorporated into Niacinamide Adenine Dinucleotide (NAD) & excreted in urine
|
|
|
What is currently the most effective drug for elevating HDL?
|
Niacin
|
|
|
List the effects of Niacin administration
|
1. decreases lipase activity in adipose tissue
-reduces free FA flux to liver -decreases hepatic synthesis of Triglycerides & VLDL -LOWER plasma LDL & TG's 2. increases ApoAI to elevate plasma HDL |
|
|
What are the therapeutic uses of Niacin?
|
1. DOC for pts with elevated LDL & lowered HDL
2. normalizes LDL when combined with Resin or Statin for treatment of Heterozygous Familial Hypercho-cholesterolemia & other Hypercholesterolemias 3. Familial combined Hyper-lipoproteinemia 4. Familial Dysbetalipoproteinemia |
|
|
DOC for patients with elevated LDL & lowered HDL
|
Niacin (B3)
|
|
|
What are the manifestations of Niacin toxicity? (5)
|
1. MC is CUTANEOUS VASODILATION with WARM SENSATION
2. pruritis, rashes, dry skin, nausea, abdominal discomfort 3. may elevate Aminotransferases but true Hepatotoxicity is rare 4. glucose tolerance may be impaired 5. may cause Hyperuricemia & Gout |
|
|
List the Fibric Acid derivatives used as lipid-lowering agents
|
Fenofibrate
Gemfibrozil Clofibrate Benzafibrate |
|
|
List the mechanism of action of the Fibrates
|
act as ligands for the nuclear transcription factor PPAR-alpha in hepatocytes
activation of hepatic PPAR-alpha DECREASES PLASMA TRIGLYCERIDES, VLDL, & LDL; increases HDL **greatest effect is on decreasing Triglycerides |
|
|
What are the Fibrates used for the treatment of?
|
Hypertriglyceridemia = hepatic overproduction of VLDL
Dysbetalipoproteinemia = deficiency of Apolipoprotein E -increased remnants = VLDL, IDL, & Chylomicrons |
|
|
What are the toxic effects of the Fibrates?
|
Rare but:
-skin rashes -GI symptoms -myopathy -arrhythmias -hypokalemia -increases Aminotransferases or Alk Phos -decreases in WBC's or hematocrit -rarely Rhabdomyolysis -increased incidence of Gallstones |
|
|
What drugs do the Fibrates potentiate?
|
Anticoagulants
-Indanedione -Coumarin (Warfarin) |
|
|
List 3 Bile Acid-binding Resins used as Lipid-lowering agents
|
1. Colestipol
2. Cholestyramine 3. Colesevelam **C(h)oles |
|
|
Describe the Bile Acid resins
|
large cationic exchange resins, insoluble in water, that bind bile acids to prevent their intestinal absorption & increase excretion
1. upregulates 7-alpha hydroxylase = hepatic enzyme that catalyzes synthesis of bile acids from cholesterol 2. increased bile acid synthesis reduces the amount of hepatic cholesterol 3. reduction in hepatic cholesterol increases LDL recpetors 4. enhances removal of LDL from circulation 5. lowers plasma LDL & elevates plasma HDL |
|
|
What are the Bile Acid Resins used therapeutically for?
|
1. Primary Hypercholesterolemia
2. Digoxin toxicity = bind digoxin |
|
|
What is the toxicity of Bile Acid Resins? (6)
|
1. no significant systemic effects b/c they are not absorbed
2. Unpleasant sandy & gritty quality 3. occasional heartburn & diarrhea 4. Bloating & constipation occur due to decreased fat absorption 5. Hypoprothrombinemia due to Vitamin K malabsorption 6. Steatorrhea in pts with Bowel Disease or Cholestasis 7. Gallstone formation is enhanced in Obese pts |
|
|
What drugs do Bile Acid Resins impair the absorption of?
|
1. Digoxin
2. Thiazides 3. Tetracycline 4. Fluvastatin 5. Thyroxine 6. Aspirin DAFTTT |
|
|
What is the name of the Cholesterol absorption blocker?
|
Ezetimibe
**"Easy time" at blocking cholesterol absorption |
|
|
What effects does Ezetimibe possess?
|
1. inhibits intestinal absorption of Cholesterol & Phytosterols
2. reduces plasma LDL with minimal HDL increase 3. effective even when there is no dietary cholesterol b/c it inhibits absorption of cholesterol excreted in bile |
|
|
What are the therapeutic used of Ezetimibe?
|
1. Primary Hypercholesterolemia
2. single daily dose of 10 mg reduces LDL by 18% |
|
|
What is the low incidence toxicity associated with Ezetimibe?
|
reversible hepatic impairment = increased LFT's
|
|
|
When are drug combinations useful?
|
1. When VLDL levels increase during Resin therapy of Hypercholesterolemia
2. when VLDL & LDL are both elevated 3. when VLDL or LDL levels are not normalized by a single drug 4. when elevated Lp(a) or HDL deficiency occurs with other hyperlipidemias |
|
|
Niacin + Lovastatin = ?
|
Advicor
|
|
|
Ezetimibe + Simvastatin = ?
|
Vytorin
|
|
-
|
-
|

