![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
66 Cards in this Set
- Front
- Back
|
What are the 2 compartments of the Adrenal Cortex and what do they secrete?
|
1. Outer Zona Glomerulosa ➡ mineralcorticoid Aldosterone
2. Inner Zona Fasciculata ➡ Cortisol 3. Reticularis ➡ Adrenal Androgens |
|
|
What regulates Aldosterone secretion from the Zona Granulosa?
|
1. extracellular K+ channels that sense Potassium levels
-Hyperkalemia = secretion 2. Angiotensin receptors |
|
|
What regulates the secretion of Glucocorticoids?
What are the 2 enzymes that catalyze Glucocorticoid production? |
ACTH
17a-hydroxylase (P450c17) 11b-hydroxylase (P450c11) |
|

-
|

-
|
|
|
What is the naturally-occuring Glucocorticoid?
What is it derived from? |
Cortisol
Cholesterol |
|
|
What 2 things are the synthesis and secretion of Cortisol tightly regulated by?
|
CNS which is very sensitive to negative feedback by:
1. circulating Cortisol 2. Exogenous (synthetic) Glucocorticoids |
|
|
__1__ mg of Cortisol is secreted daily following a Circadian rhythm regulated by __2__
3. What is the half-life of Cortisol? |
1. 10-20
2. ACTH 3. 60-90 min |
|
|
What is 90% of circulating Cortisol bound to?
What about the other 5-10%? |
Corticosteroid binding globulin (CBG) = synthesized by Liver
Free or loosely bound to Albumin |
|
|
How is Cortisol inactivated?
|
Mostly in the Liver
1. ~ 20% is converted to Cortisone 2. only 1% is excreted in urine |
|
|
Describe the Glucocorticoid receptors
|
Primarily Cytoplasmic, in the form of Oligomeric complexes with 2 molecules of Heat Shock Proteins (Hsp90)
|
|
|
List the 2 types of Glucocorticoid "orpha receptors"
|
Type 1 = mineralcorticoid receptor; expressed mainly in organs of excretion such as kidney, colon, salivary glands, sweat glands
Type 2 = Glucocorticoid receptor; broader tissue distribution |
|
|
Describe the Steroid-Receptor Interaction for Cortisol
|
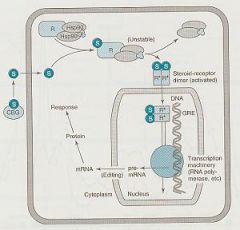
1. free steroid enter cell cytoplasm to release receptor from Hsp90
2. Steroid-receptor complex enters nucleus 3. S-R binds to the Glucocorticoid Response Element (GRE) 4. Regulates tsc by RNA polymerase and other factors 5. mRNA edited and exported to cytoplasm to form protein |
|
|
What % of expressed genes do Glucocorticoids regulate in any given cell?
|
10-20%
|
|
|
What are Glucocorticoid effects mainly due to?
|
Proteins synthesized from mRNA transcribed by their target genes
|
|
|
What are the 3 main effects Glucocorticoids produce?
|
1. Anti-growth
2. Anti-inflammatory 3. Immunosuppressive effects |
|
|
Most of the effects of Corticosteroids (Cortisol) are __1__, while others are __2__
|
1. direct
2. "permissive" = effects that required glucocorticoid presence such that the fxn becomes deficient when glucocorticoid is absent |
|
|
Glucocorticoids induce the synthesis of __1__, an inhibitor of __2__
|
1. Lipocortin
2. Phospholipase A2 **inhibits Prostaglandin and Leukotriene synthesis = Anti-inflammatory |
|
|
Glucocorticoids inhibit the production of __1__, which inhibits the proliferation of __2__
|
1. IL-2
2. T lymphocytes **Anti-inflammatory |
|
|
Glucocorticoids inhibit the release of these 2 things from Mast cells and Platelets
|
1. Histamine = decreases capillary permeability (usually causes permeability)
2. Serotonin = less platelet aggregation (usually causes aggregation) |
|
|
How do Corticosteroids reduce the # of Lymphocytes, Monocytes, Eosinophils, and Basophils?
|
causes them to move from the Vascular bed to Lymphoid tissues
|
|
|
List the Metabolic effects Glucocorticoids (Corticosteroids) possess
|
1. Stimulate Gluconeogenesis and Glycogen synthesis
2. release Amino Acids during muscle catabolism = provide AA's to liver for Gluconeogenesis 3. Stimulate hormone-sensitive Lipase & Lipolysis = provides more glycerol to liver for gluconeogenesis 4. Elevate serum Glucose to stimulate Insulin release BUT inhibit glucose uptake by muscle cells = allows more delivery to brain |
|
|
What is the ultimate purpose of the Metabolic effects of Cortisol?
|
Supply adequate glucose to the brain in the fasting state
|
|
|
Where do the Catabolic & Anabolic effects of Glucocorticoids occur?
|
1. Lymphoid tissue
2. Connective tissue 3. Muscle 4. Fat 5. Skin |
|
|
What effects do supraphysiologic amounts of Corticosteroids have on muscle, skin, and bone?
|
Reduce muscle mass causing weakness
Skin thinning Osteoporosis (in Cushing's syndrome) |
|
|
Reduced growth in children on Corticosteroids can be partly prevented with what?
|
high doses of Growth Hormone
|
|
|
What effects do Glucocorticoids have on the CNS?
|
1. marked slowing of EEG alpha rhythm
2. behavioral disturbances initially as insomnia & euphoria followed by depression 3. large doses may increase Intracranial pressure 4. suppressed Pituitary release of ACTH, GH, TSH, LH |
|
|
Describe how Cortisol maintains vascular responsiveness to catecholamines
|
cortisol up-regulates Alpha1-receptors on arterioles, increasing vasoconstrictor effect of Norepinephrine
|
|
|
How could Glucocorticoids contribute to Peptic Ulcers?
|
by suppressing local immune response to H. pylori
|
|
|
List 3 miscellaneous effects of Corticosteroids
|
1. promote fat redistribution to increase visceral, facial, nuchal and supraclavicular fat
2. antagonize Vitamin D effect on intestinal Calcium absorption 3. increase numbers of platelets and RBC's |
|
|
What effect does Cortisol deficiency have on renal function?
|
Impairs renal function and augments (increase) Vasopressin (ADH) secretion resulting in an inability to excrete a water load normally
**Vasopressin (ADH) causes increased water reabsorption = decreased urination volume |
|
|
When would Glucocorticoids be given to fetuses?
|
When they are premature = stimulate structural and functional development of fetal lungs including the production of Surfactant
|
|
|
List the names of commonly used Glucocorticoids (6)
|
1. Hydrocortisone
2. Prednisone 3. Triamcinolone 4. Betamethasone 5. Dexamethasone 6. Beclomethasone |
|
|
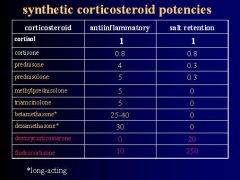
How are the synthetic Corticosteroids traditionally grouped? What are their actions?
|
Glucocorticoids = affect glucose metabolism & inflammation
Mineralcorticoids = affect Na+ or Salt retention **the 2 groups of effects are usually not closely related & reflect distinct actions at 2 distinct receptors |
|
|
2 steroids classified as Glucocorticoids but also have some Mineralcorticoid activity
|
Cortisol
Prednisone |
|
|
Steroid classified as a Mineralcorticoid but also affects Glucose metabolism (Glucocorticoid)
|
Fludro-cortisone
|
|
|
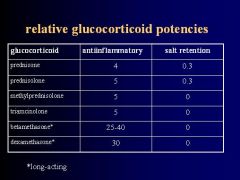
2 Steroids that have strong anti-inflammatory action but no salt-retaining activity
|
Beta-methasone
Dexa-methasone **are also long-lasting |
|
|
Steroid that has Salt-retaining activity but no anti-inflammatory activity
|
Des-oxy-cortico-sterone
|
|
|
What steroids should be used whenever Electrolyte Abnormalities are present?
|
Corticosteroids without Salt-retaining activity
-Methylprednisolone -Triamcinolone -Betamethasone -Dexamethasone |
|
-
|

-
|
|
-
|
-
|
|
|
What are the 2 Corticosteroids that have potent Salt-retention activity?
|
Des-oxy-cortico-sterone
Fludro-cortisone |
|
|
2 Corticosteroids that have high Anti-inflammatory activity but no Salt-retention activity
|
Beta-methasone
Dexa-methasone **both are long-acting |
|

-
|
-
|
|
|
What is the treatment regimen for Adrenocortical Insufficiency (Addison's disease = adrenal atrophy)
|
Life-long combined glucocorticoid and mineralcorticoid; use HYDROCORTISONE + Mineralcorticoid (Fludro-cortisone)
|
|
|
What should you not use to treat Adrenocortical Insufficiency (Addison's disease)?
|
Glucocorticoids that have no salt-retaining activity
-Methylprednisolone -Triamcinolone -Betamethasone -Dexamethasone |
|
|
1. What is the cause of Bilateral Adrenal Hyperplasia (Cushing's disease)?
2. List the main characteristics 3. What is the treatment? |
1. ACTH-secreting pituitary adenoma
2. moon facies, trunk obesity, muscle wasting, purple striae, skin bruising, osteoporosis 3. large dose Hydrocortisone following surgical removal of tumor |
|
|
Condition referred to as "iatrogenic complication of long-term corticosteroid therapy"
|
Cushing's syndrome
|
|
|
What is used to treat Aldosteronism (aldosterone excess resulting from Adrenal Adenoma)
|
Spirono-lactone
|
|
|
Describe the "Dexamethasone Suppression Test"
|
used to diagnose Cushing's disease
-high cortisol levels are reduced to 50% by Dexamethasone |
|
|
Are glucocorticoids curative or not curative in Nonadrenal disorders?
|
Not curative, and whenever possible should be used only to supplement specific treatment for the disease
|
|
|
What are the most commonly used Glucocorticoids used to treat NONADRENAL disorders? (6)
|
1. Beta-methasone
2. Dexa-methasone 3. Hydrocortisone 4. Prednisolone 5. Methyl-prednisolone 6. Triam-cinolone |
|
|
What 3 principles should be kept in mind when using Glucocorticoids to treat CHRONIC Nonadrenal disorders?
|
1. procede carefully with LOW DOSES
2. Intermittent administration (i.e. alternate days) 3. DO NOT DISCONTINUE ABRUPTLY |
|
|
What can minimize systemic side-effects when giving Glucocorticoids?
|
Local administration
-hydrocortisone by enema for Ulcerative Colitis -inhaled steroids for asthma |
|
-
|

-
|
|
|
Toxicity of Synthetic Glucocorticoids:
When used for less than __1__ serious side-effects are infrequent, but these 3 things may occur: __2__ |
1. 2 weeks
2. Insomnia, Behavioral changes, Acute peptic ulcers |
|
|
List the major side effects of Synthetic Glucocorticoids that may occur if given for long periods of time
|
1. Metabolic side-effects resulting from the hormonal actions = Iatrogenic Cushing's Syndrome
-moon facies, truncal obesity, punctate acne, muscle wasting, skin thinning, osteoporosis, diabetes 2. Peptic ulcer, myopathy, acute psychosis, depression, glaucoma, HTN, Fluid retention & edema 3. Adrenal suppression resulting in Anorexia, nausea or vomiting, weight loss, lethargy, headache |
|
|
In what patients should caution be used when giving Synthetic Glucocorticoids? (8)
|
1. Peptic ulcer
2. Heart disease 3. HTN (up-regulate Alpha-1 receptors on arterioles) 4. Psychoses 5. Infections 6. Diabetes 7. Osteoporosis 8. Glaucoma |
|
|
The most important mineralcorticoid in man that is synthesized in the Zona Glomerulosa
|
Aldosterone
|
|
|
Where does Aldosterone bind?
|
receptors in:
1. Distal Convoluted tubule 2. Proximal Collecting tubules Promotes Na+ reabsorption |
|
|
What is the precursor of Aldosterone that is also normally synthesized and secreted?
|
Deoxy-cortico-sterone
|
|
|
Most commonly prescribed Mineralcorticoid; has potent Glucocorticoid and Mineralcorticoid activity
|
Fludro-cortico-sterone
|
|
|
Mineralcorticoid that is used to treat Adrenocortical insufficiency associated with Mineralcorticoid deficiency
|
Fludro-cortico-sterone
|
|
|
SELECTIVE INHIBITOR of STEROID SYNTHESIS commonly used for testing adrenal function
|
Mety-rapone
|
|
|
Nonselective inhibitor of Adrenal & Gonadal steroid synthesis used for treatment of Cushing's Disease
|
Ketoconazole
|
|
|
Glucocorticoid antagonist with strong Anti-progestin activity and has been used as an Abortifacient?
|
Mife-pristone
|
|
|
Aldosterone antagonist widely used to prevent Hypokalemia in treatment of HTN and Congestive Heart Failure
|
Spironolactone
|

