![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
132 Cards in this Set
- Front
- Back
|
List 4 ways cells can be resistant to Alkylating agents
|
1. Impermeable to drug, pump drug out
2. Alternate targets for drug (Glutathione = reacts with agent) 3. Increased DNA repair 4. No Apoptosis = loss of p53 |
|
|
Adverse Effects of Alkylating Agents:
1. Target these 3 rapidly growing cells 2. Dose-dependent toxic effect causes decreased ____ and ___ 3. Maximum suppression is ______ after therapy 4. Recovery is ______ after drug 5. Monitor patient tolerance by _____ and ____ |
1. Bone Marrow, GI tract, Spermatogenesis
2. Leukocytes and Platelets 3. 10 days to 4 weeks 4. 3 to 6 weeks 5. CBC and Hematocrit |
|
|
Secondary Malignancy:
Most chemical carcinogens act by _______ of nuclear DNA, leading to altered structure and function |
Covalent modification
***Alkylating agents |
|
|
The concern of Secondary Malignancy is Proportional or Inversely Proportional to the age of the patient?
|
Inversely Proportional = the younger the patient, the more concern
|
|
|
Alkylating agent that is a nitrogen mustard and was the 1st alkylating agent
|
Mechlorethamine
|
|
|
Alkylating agent that is only administered IV b/c it produces blisters and is caustic to skin and mucous membranes
|
Mechlorethamine
|
|
|
How is Mechlorethamine often administered to target its site?
|
In the arterial supply directly to the tumor
|
|
|
Alkylating agent whose half-life is several minutes, reacts and breaks down rapidly in water and salt.
Used to treat Hodgkin's Lymphoma |
Mechlorethamine
|
|
|
What are the acute adverse effects of Mechlorethamine?
|
Nausea and vomiting
|
|
|
What are the delayed adverse effects of Mechlorethamine?
|
Decreased blood counts
- minimum levels 10-12 days after administration - Recovery = 3-6 wks |
|
|
What is the primary use of Mechlorethamine?
|
Hodgkin's disease
**part of MOPP -Mechlorethamine -Oncovin = Vincristine (VCR) -Procarbazine -Prednisone |
|
|
What are the 2 Nitrosourea alkylating agents?
|
Carmustine (BCNU)
Lomustine (CCNU) |
|
|
Alkylating agents that are activated NON-ENZYMATICALLY, are LIPID-SOLUBLE and CROSS the BBB
Primary use is BRAIN TUMORS |
Nitrosureas
-Carmustine -Lomustine |
|
|
Why are the Nitrosureas primarily used for Brain Tumors?
|
because they are lipid-soluble and can cross the BBB
|
|
|
How are the Nitrosureas administered?
|
IV or Direct Implant
|
|
|
What are the 2 adverse effects of Nitrosureas?
|
GI
Myelosuppression |
|
|
One of the most widely used Alkylating agents mostly b/c it can be given ORALLY
|
Cyclophosphamide
*also given via IV |
|
|
Alkylating agent that is not intrinsically active, but must be activated by Liver P450's = good ORAL drug
|
Cyclophosphamide
|
|
|
What are the acute adverse effects of Cyclophosphamide?
|
Nausea and vomiting
|
|
|
Alkylating agents with these ADR's:
1. Bone Marrow depression (moderate) 2. Alopecia 3. Sterile Hemorrhagic Cystitis |
Cyclophoshamide
|
|
|
Metabolic product of Cyclophosphamide that causes Sterile Hemorrhagic Cystitis
|
Acrolein = reactive and water soluble molecule that gets excreted in urine
- reacts with components of the epithelium and you get bleeding |
|
|
What are 2 things that can be done to prevent Sterile Hemorrhagic Cystitis with Cyclophosphamide?
|
1. Over-hydrate before giving drug = quicker washing out when given
2. Mesna = thiol which reacts with Acrolein in urine |
|
|
Bifunctional "platinating agent" = cross-links DNA
|
Cis-Platin
-binds to 2 different bases and cross-links them |
|
|
Cisplatin:
-Administered? -Excreted? |
1. IV
2. Urine |
|
|
Cisplatin is relatively non-toxic to ________
|
bone marrow
|
|
|
What are the 2 acute adverse effects of Cisplatin and what is done to minimize them?
|
1. SEVERE nausea and vomiting = use HT3 antagonists
2. RENAL Toxicity = ensure adequate hydration |
|
|
Platinum complex that causes:
-less nausea and renal toxicity than Cisplatin -but more myelosuppression |
Carboplatin
|
|
|
Platinum complex that is has less renal toxicity than Cisplatin, but is neurotoxic
|
Oxaliplatin
|
|
|
Alkylates DNA and also causes strand scission (separation) = blocks DNA and RNA synthesis
Strongly Leukemogenic and Teratogenic |
Procarbazine
|
|
|
Procarbazine shows "standard" alkylating toxicities (NVD, bone marrow depression), but it also has these adverse effects
|
1. Leukemogenic = causes leukemia
2. Teratogenic = causing malformation of fetus |
|
|
What is the mechanism of Anti-cancer Anti-metabolites?
|
-Analog of a normal component of the target cell
-enters into a normal metabolic pathway, but then BLOCKS that pathway |
|
|
Anticancer antimetabolite that is a Dihydrofolate Reductase (DHFR) substrate and inhibitor
|
Methotrexate = Folic acid analog
|
|
|
What are the 2 advantages of Methotrexate being polyglutaminated in mammalian cells?
|
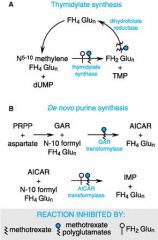
1. can help it stay in the cells
2. there are steps that are more effectively inhibited by methotrexate derivatives |
|
|
What does Methotrexate decrease the synthesis of?
|
THF and therefore Thymidine = decreased DNA and protein synthesis
|
|
|
Methotrexate:
1. Administration 2. Excretion 3. Cell cycle phase specificity |
1. Orally, IV, Intrathecally
2. Urine 3. S phase |
|
|
Describe "rescue therapy" using Methotrexate
|
Give high dose to have maximum effect on tumor cells, "rescue" normal cells by giving Folinic Acid (Citrovorin, Leucovorin)
|
|
|
What cell cycle phase is Methotrexate only effective in?
|
S phase
|
|
|
Folate antagonist that with chronic use can cause Hepatotoxicity = fatty liver
|
Methotrexate
|
|
|
What are the 2 Folinic Acid drug names that can be given in Methotrexate "rescue"?
|
Citro-vorin
Leuco-vorin |
|
|
What are 3 ways tumors can become resistant to Methotrexate?
|
1. decreased drug accumulation
2. amplified DHFR 3. altered DHFR |
|
|
What are the 2 Antimetabolite Purine Antagonists?
|
1. 6-mercaptopurine
2. 6-thioguanine |
|
|
6-MP and 6-TG:
1. Administration? 2. Well-tolerated, but this happens only at high doses |
1. Oral
2. Bone Marrow depression |
|
|
Purine antagonist that inhibits AMP and GMP synthesis = blocks DNA and RNA synthesis
|
6-Mercaptopurine
|
|
|
Anti-tumor Purine antagonist that is incorporated into RNA and DNA, altering function
|
6-thioguanine
|
|
|
The 2 ways tumor cells become resistant to these 2 drugs is by:
1) a decrease in HPRT activity 2) increase in Alkaline Phosphatase |
6-Mercaptopurine
6-Thioguanine |
|
|
What are the 2 Antimetabolite Pyrimidine Antagonists?
|
5-fluorouracil
Cytarabine |
|
|
T or F: Purine analogs are far more toxic than Pyrimidine analogs
|
False: Pyrimidine analogs are more toxic = Bone Marrow, GI
|
|
|
Pyrimidine Antagonist that inhibits Thymidylate Synthase (inhibits conversion of dUMP to dTMP)
|
5-Fluorouracil
|
|
|
What is 5-Fluorouracil's activity enhanced by?
|
Folinic Acid (Leukovorin)
*Leukovorin also "rescues" Methotrexate toxicity |
|
|
Cytosine analog, chain terminator used as an anti-cancer drug
|
Cytarabine
|
|
|
Anti-tumor Antimetabolites that is bioactivated by HGPRTase
|
6-Mercaptopurine
|
|
|
What is the connection between Flucytosine and 5-fluorouracil?
|
Flucytosine is bioactivated into 5-FU in Fungal cells = inhibit Thymidylate synthase
|
|
|
What are the 2 Vinca Plant Alkaloid drugs?
|
1. Vinblastine
2. Vincristine |
|
|
What are the Vinca Alkaloids isolated from?
|
Vinca Rosea = Periwinkle
|
|
|
What is the mechanism of action of the Vinca Alkaloids?
|
Inhibit/reverse tubulin polymerization = disrupts mitotic spindles
|
|
|
What do Vinca Alkaloids cause?
|
Metaphase Arrest
|
|
|
Vinca Alkaloids:
1. Administration? 2. Excretion? |
1. IV = due to large size
2. Biliary excretion |
|
|
What is Vinblastine's adverse effect?
|
1. NVD
2. Alopecia 3. Bone marrow depression *vinBLASTin BLASTs Bone marrow |
|
|
Why is Vincristine's use limited to short duration?
|
Peripheral neuropathy = MT's that we are blocking for therapeutic effect are also blocking transport within the long axons of nerves
|
|
|
What are the 2 Podophyllotoxin Plant Alkaloids?
|
1. Etoposide (VP-16)
2. Teniposide **-POSIDE's |
|
|
What is Etoposide/Teniposide's mechanism of action?
|
Topoisomerase II inhibitors = end up with DNA molecules with double strand breaks that are pretty much impossible to repair
Also marks DNA for degradation |
|
|
What stage does Etoposide/Teniposide arrest cells in?
|
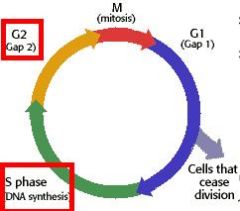
S-G2 stage
|
|
|
Etoposide/Teniposide:
1. Administration 2. Adverse effects |
1. Oral and IV
2. N/V, Alopecia, BM suppression |
|
|
What are the 2 Camptothecin Plant Alkaloids?
|
Top-otecan
Irin-otecan |
|
|
Anti-tumor agents that are Topoisomerase I inhibitors
|
Topotecan & Irinotecan
**-TECAN's = TOPO I inhibitors |
|
|
Topotecan/Irinotecan:
Administration? |
IV
|
|
|
Plant Alkaloids these ADR's:
-Mucositis -Severe diarrhea -Cholinergic syndrome = salivation, cramping***which one specifically? |
Topotecan
Irinotecan --> Cholinergic syndrome |
|
|
What are the 2 Taxane Plant Alkaloids?
|
1. Paclitaxel (Taxol)
2. Docetaxel (Taxotere) |
|
|
Antitumor agents that STABILIZE microtubules so that the mitotic spindle cannot break down
|
Paclitaxel & Docetaxel
**-TAXOL's |
|
|
What phase of the cell cycle are -Taxel's specific for?
|
M phase
|
|
|
What are the acute adverse effects of -Taxels?
|
Hypersensitivity
Nausea *probably due to Cremophor |
|
|
What are the delayed adverse effects of Taxanes?
|
BM suppression
Some neuropathy |
|
|
Antitumor agents that are administered via IV in Cremophor (solubilizing agent) and show promise against solid tumors
|
Paclitaxel & Docetaxel
|
|
|
What are most antitumor antibiotics produced by?
|
Microbes -> Streptomyces
|
|
|
Antitumor antibiotics interact with DNA and/or RNA, but most do not ______
|
alkylate
|
|
|
How are all Antitumor Antibiotics administered?
|
IV
|
|
|
What drugs are in the Anthracycline antitumor antibiotics category
|
-rubicin
Doxorubicin, Daunorubicin |
|
|
Anti-tumor Antibiotics that:
Intercalate into DNA and slide between adjacent bases = block Topoisomerase II, inhibit DNA and RNA synthesis, cause strand breaks Generate free radicals |
-Rubicin's = Daunorubicin & Doxorubicin
|
|
|
How are the -rubicin's cleared?
|
Metabolized in liver
|
|
|
The "signature" ADR of these drugs is CARDIOTOXICITY
|
Daunorubicin & Doxorubicin
= -Rubicin's |
|
|
What is the Cardiotoxicity of Anthracyclines a function of?
|
Cumulative dose
|
|
|
Cardiotoxicity of Anthracyclines:
-may be exacerbated by __1__ -complications: __2__ -mechanism: __3__ |
1. radiation
2. arrhythmias, cardiomyopathy, CHF 3. free radicals |
|
|
What is the Cardiotoxicity of Anthracyclines minimized by?
|
Dexrazoxane
|
|
|
Antitumor antibiotic that is a mixture of glycopeptides that complexes with Fe and O2 -> generates free radical -> causes DNA strand breaks
|
Bleomycin
|
|
|
When is Bleomycin only active (what cell cycle phase)?
|
G2 = synthesis of components needed for mitosis
|
|
|
ADR's of this antitumor antibiotic are:
1. Hypersensitivity 2. Cutaneous rxns 3. PULMONARY FIBROSIS |
Bleomycin
|
|
|
Antitumor antibiotic that is particularly effective against SOLID TUMORS
-its activation is favored by Hypoxia -Alkylates DNA |
Mitomycin C
|
|
|
How is Mitomycin C administered?
|
IV or by Bladder instillation
|
|
|
Antitumor antibiotic that intercalates in DNA and blocks RNA and DNA synthesis
-Used for Wilms's tumor, Ewing's Sarcoma, and Rhabdomyosarcoma |
Dactinomycin (Actinomycin D)
**Actinomycin D is used for childhood tumors -> children ACT out |
|
|
ADR of this antitumor antibiotic is: inflammation at sites of prior radiation = "radiation recall"
|
Dactinomycin
|
|
|
What is the function of Adrenocorticosteroids, Hydrocortisone, and Prednisone in treating cancer?
|
suppresses proliferation of immune cells (leukocytes, lymphocytes) = used in therapy for leukemias and lymphomas
|
|
|
How are the Adrenocorticosteroids, Hyrdocortisone, Prednisone administered?
|
Orally
|
|
|
What are the delayed adverse effects of Adrenocorticosteroids, Hyrdocortisone, Prednisone?
|
Fluid retention
Immunosuppression Diabetes |
|
|
Blocks conversion of androgens to estrogens, specific for estrogen production
|
Aromatase inhibitors
|
|
|
List the 3 Aromatase Inhibitors
|
1. Anastrazole
2. Letrozole 3. Exemestane |
|
|
Aromatase inhibitors more effective against the Peripheral Aromatase form
-used in treatment for Estrogen receptor primary and metastatic breast cancer |
Anastrazole
Letrozole **-AZOLE's |
|
|
Steroidal irreversible aromatase inhibitor
|
Exemestane
*no cross-resistance with azoles |
|
|
What are the acute and delayed adverse effects of Exemestane?
|
Acute = mild nausea, headache
Delayed = Fatigue, hot flushes |
|
|
Selective Estrogen Receptor Antagonist (SERM) that is an antagonist in the breast but an agonist in Endometrium, increasing the risk of Endometrial Cancer
|
Tamoxifen
|
|
|
SERM that is an antagonist in the breast, but also an AGONIST in Bone, which may work against Osteoporosis
|
Raloxifene
|
|
|
Describe the mechanism of SERM's
What group of women are they more effective in? |
Competing ligands for Estrogen Receptor, but don't activate it
Post-menopausal women |
|
|
What is Tamoxifen used for to treat?
|
ER+ Primary and Metastatic breast cancer
|
|
|
List 3 adverse effects of the SERM's
|
1. Nausea
2. Hot flashes 3. Vaginal bleeding |
|
|
Name the two SERM's
|
Tamoxifen
Raloxifene *-oxifen(e) |
|
|
List the 2 Androgen Receptor Antagonists
|
Flutamide
Bicalutamide *"-lutamide's" |
|
|
2 drugs used with radiation to treat Prostate Cancer
|
Flutamide
Bicalutamide |
|
|
What are the adverse effects of Flutamide and Bicalutamide?
|
Mild:
-nausea -hot flashes -transient hepatic effects |
|
|
Explain why Asparaginase might be used in anti-cancer therapy
|
Depletes serum Asparagine, which is needed in large amounts by some tumors
|
|
|
What cancer is Asparaginase specifically used against?
|
Acute Lymphocytic Leukemia (ALL)
**aSPARE ALL*** |
|
|
Asparaginase:
1. Administration? 2. Adverse effect |
1. Parenteral
2. Hypersensitivity (can be fatal!) |
|
|
Antitumor agent that inhibits Ribonucleotide Reductase
|
Hydroxyurea
|
|
|
Antitumor agent that is used to treat Leukemias and SCC of the head and neck. Also has these 4 ADR's:
1. N/V 2. Mucositis 3. Rash 4. BM suppression |
Hydroxyurea
|
|
|
Interferon-alpha:
-polypeptide cytokine produced by __1__ -__2__ administration -alters __3__, __4__, and __5__ |
1. White blood cells
2. Parenteral 3. gene expression 4. antiviral 5. immnomodulatory |
|
|
Antitumor agent used against these 3 tumors:
1. Hematologic malignancies 2. Metastatic Melanoma 3. Renal cell carcinoma |
Interferon-alpha
|
|
|
What are the adverse effects of IFN-alpha?
|
1. Fever and chills
2. anorexia 3. weakness |
|
|
Monoclonal antibody against VEGF-A, a critical angiogenic growth factor
|
Bevacizumab (Avastin)
**Bev-VEGF |
|
|
Bevacizumab (Avastin) is combined with __1__ as a first-line therapy for metastatic __2__
|
1. Fluoropyrimidine
2. Colorectal cancer |
|
|
Antitumor agent with these ADR's:
1. HTN 2. Thromboembolic events 3. wound healing complications 4. GI perforations |
Bevacuzimab = Ab to VEGF-A
|
|
|
How is Bevacizumab administered?
|
IV
|
|
|
Monoclonal antibody against HER2neu/ErbB-2 oncogene product (Epidermal Growth Factor receptor)
|
Trastuzumab (Herceptin)
|
|
|
What is Trastuzumab used against?
|
Breast cancer whose cells overexpress HER-2
|
|
|
Antitumor agent with these ADR's:
1. Infusion rxns 2. Hypersensitivity (b/c we are giving a protein product) 3. **Cardiomyopathy** |
Trastuzumab
|
|
|
How is Trastuzumab administered?
|
IV
|
|
|
What 2 cancers is Imatinib (STI-571, Gleevec) used against?
|
1. Chronic Myelogenous Leukemia (CML)
2. GI Stromal tumors (GIST) |
|
|
Antitumor agent that inhibits these 2 things:
Bcr-Abl tyrosine kinase in CML Kit kinase in GIST |
Imatinib (Gleevec)
|
|
|
How is Imatinib (Gleevec) administered?
|
Orally
|
|
|
What are 3 adverse effects of Imatinib (Gleevec)?
|
1. Myelosuppression
2. ***Edema and fluid retention*** 3. Hepatotoxicity |
|
|
What does Gefitinib (Iressa) inhibit?
|
EGF-R tyrosine kinase
|
|
|
Gefitinib:
1. Administration 2. Adverse effects |
1. Orally
2. Fever, Dyspnea |
|
|
What cancer is Gefitinib used against?
|
NON-Small cell lung cancer
*Jeff S. has a non-small cell lung CA |
|
|
Signal Transduction Inhibitors transform cancer from a "__1__" disease to a "__2__" disease
|
1. curable
2. manageable |
|
|
What are the 2 Signal Transduction Inhibitors?
|
Imatinib
Gefitinib |

