![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
166 Cards in this Set
- Front
- Back
|
Na channels
|
targets for local anesthetics, antiepileptic drugs, antidysrhymics
|
|
|
Gate Theory
|
ion channel exists in one of 3 states:
Resting, Active, Inactive -state depends on membrane potential |
|
|
Ca, Mg ATPase
|
-plasma membrane pump (Ca extrusion)
-sarcoplasmic reticulum pump (Ca sequestration) -activated by Ca2+-calmodulin -indirect activation by cGMP, cAMP |
|
|
phospholamban
|
important protein in SR for Ca2+ pump activity
-phosphorylated by A-Kinase = dissoc from Ca pump = more Ca sequestered in SR = ↑ contract |
|
|
examples of indirect activation of Ca, Mg ATPase pump
|
*phosphorylation of phospholamban by A-Kinase in cardiac SR
*nitrovasodilators ↑ cGMP = ↑ pump out of cell = ↓ contract |
|
|
H, K-ATPase
|
target for gastric secretion inhibitors (ex. omeprazole)
-mediates acid secretion from parietal cell |
|
|
omeprazole
|
-inactive at neutral pH
-at acid pH6 forms active species which covalently binds to pump |
|
|
Ca Channels
|
target for: antihypertensives, antianginals, antidysrhymthmics
two types: L-type (long-lasting) channel and receptor-operated Ca2+ channel |
|
|
L-type Ca2+ channel
|
drug receptor sites α1 subunit
-voltage-dependent, inactivates slowly in A state -sites for phosphorylation (ex. NE inotropic via cAMP kinase) |
|
|
prototype blockers of L-type channels
|
nifedipine (distinct binding site), verapamil, diltiazem
-either slows recovery or prev. Ca2+ entry during A state |
|
|
receptor-operated Ca2+ channels
|
-unknown structure, allows Ca2+ entry with no change in MP
-?modif of L-typ by agonist -?separate channel pop'n ex. isolated blood vessel exp |
|
|
isolated blood vessel experiment
|
studied mechanism of receptor-operated Ca2+ channel
-found that even after full depolarization, could further contract w/ NE |
|
|
K+ channels
|
-diverse properties and reg.
-targets for oral hypoglycemics, class III antidysrhythmics, vasodilator K+ channel openers -classified based on gating, conductance, pharmacology |
|
|
voltage-gated K+ channel
|
ex. phase 3 of action potentials
|
|
|
ion-gated K+ channel
|
ex. Ca2+-activated K+ channels
-vary in conductance, function to terminate excitatory processes caused by ↑ Ca2+ |
|
|
Ligand-gated K+ channel
|
ex. atrial hyperpolarization by ACh
ex. ATP-dependent K+ channels (blocked by sunfonylurea-type drugs) |
|
|
ATP-dependent K+ channels
|
ligand-gated K+ channel
close when internal [ATP] ↑ = depolarization, Ca2+ entry, ↑ insulin secretion from β cells |
|
|
atrial hyperpolarization by ACh
|
ligand-gated K+ channel
ACh activ specific G protein = α subunit of Gpro activates K+ channel = ↑ K+ efflux |
|
|
3 ion channels
|
Na+, K+, ,Ca2+
|
|
|
3 ion pumps
|
1. Na, K-ATPase
2. Ca, Mg-ATPase 3. H, K-ATPase |
|
|
Na, K-ATPase
|
3 Na in = 2 K out
-target for: digitalis -electrogenic (adds -5mV to resting MP, net charge leaving) -activity varies during AP |
|
|
3 ion exchangers
|
1. Na-Ca exchange
2. Na, K, 2Cl cotransport 3. Other Ion transporters in Kidney |
|
|
Na-Ca Exchange
|
-electrogenic, 3 Na+ in = 1 Ca out, driving force: Na+ grad
-helps remove Ca from myocytes after card contract -affected by card. glycosides |
|
|
Na, K, 2Cl Cotransport
|
target for: loop diuretics
-mediates ion reabsorption in thick ascending limb of loop of Henle |
|
|
Other Ion Transporters in Kidney
|
NaH CO3 cotransport
NaCl cotransport (inhib with thiazide diuretics) NaH exchange (proximal tubule) → Na+ gradient driving force |
|
|
local anesthesia
|
loss of sensation confined to a discrete area of the body
-block in sensory nerve conduction |
|
|
methods of local anesthesia
|
*physical (trauma, hypothermia, anoxia)- potent. irreversible
*chemical (alcohol, phenol) potentially irreversible *drugs (LA) reversible |
|
|
classification of local anesthetics
|
*duration of action (short = 1 hour, long = 3 hours)
*structure: esters or amides |
|
|
examples of local anesthetics
|
procain, lidocain, mepivacaine, bupivacaine, tetracaine, ropivacaine, cocaine
|
|
|
pKa of local anesthetics
|
*all salts of weak bases
pKa > physiol pH = mainly ioniz. *non ionized crosses nerve memb., ionized binds to receptor |
|
|
mechanisms of local anesthetics
|
bind to specific receptor in Na channels
-stabilize channel in "inactivated-closed" state =no depol = no prop of AP |
|
|
onset of action of local anesthetics
|
determined by pHa and tissue pH (ie. amount of drug that can cross membrane)
-can be increased by alkalinization |
|
|
potency of local anesthetics
|
related to lipid solubility (how readily crosses membrane)
|
|
|
duration of action of local anesthetics
|
determined by protein binding (more bound = longer duration)
-affected by blood flow to area -may be increased with vasoconstrictors |
|
|
absorption of local anesthetics
|
-dep on mode of administration (topical vs injection)
-vasodilator activity (lidocaine vs cocaine = VC = arrhythmias) -vasoconstrictor addition |
|
|
distribution of local anesthetics
|
highest at site of application
-distributes to all tissues -crosses BBB and placenta |
|
|
metabolism of local anesthetics
|
amides: liver enzymatic metabolism
esters: hydrolyzed in plasma |
|
|
toxicity of local anesthetics
|
related to: plasma concentration
-drugs (lidocaine vs bupivacine) -pregnancy (lower safety margin, fetal acidosis = higher fetal:mother concentration) |
|
|
types of toxicity for local anesthetics
|
*high plasma level (OD, inject)
*allergy: esters <rare amides, more b/c of preservatives *site of injection (inadvertent spinal) |
|
|
prevention of toxicity for local anesthetics
|
-limit dose
-minimize aborption (aspirate on syringe, vasoconstrictors) -prophylaxis: benzodiazepine |
|
|
clinical use of local anesthetics
|
*skin (EMLA cream), airway
*infiltration *block: peripheral nerve, plexus, intravenous (Bier), central neural (spinal, epid.) |
|
|
components of anaesthesia
|
1.hypnosis 2.analgesia 3.amnesia 4. blunted autonomic responses
5. +/- muscle relaxation |
|
|
the ideal anethetic
|
rapid induction/recovery, changes in depth
relaxation of muscles, wide safety margin, no tox, cheap, non explosive |
|
|
classification of general anesthetics
|
*inhalational
*intravenous |
|
|
inhalational general anesthetics
|
-gases: N2O, cycloropane, xenon
-volatile liquids: halothane, isoflurane, sevoflurane, desfluran, ether, methoxyflurane, chloroform |
|
|
intravenous general anesthetics
|
-barbituates (sodium propophol)
-benzodiazepines -ketamine -methohexital |
|
|
mechanisms of general anesthetics
|
*global depression of CNS and other tissues incl. RAS and macromolecules
|
|
|
reticular activating system
|
centre of consciousness, depressed by general anesthetics
|
|
|
central role of cell membranes in general anesthetics
|
*lipid solubil. (Meyer-Overton)
*critical volume *fluidization *pressure reversal |
|
|
structure of volatile anesthetics
|
fluorinated hydrocarbons
|
|
|
potency of general anesthetics
|
Minimum Alveolar Concentration
-determined exp: conc = 50% mice don't react to tail clamp -50% patients: 0.75% halothane, 105% NO |
|
|
signs and stages of general anesthetics
|
1. loss of consciousness
2. delerium (↑ BP, HR, tone) 3. surgical (4 planes, ↑ resp depression, ↓ reflexes) 4. resp. paralysis |
|
|
delivery of general anesthetics
|
inhale through anesthetic apparatus → airway, lungs → blood uptake → tissue blood flow
|
|
|
effects common to inhalational anesthetics
|
*alter breathing pattern, depress vent response to CO2
*vasodilation, myocardial depr. *skeletal musc relaxation *uterine relaxation |
|
|
halothane
|
a general anesthetic
regular potency (in 50% patients) = 0.75% *liver toxicity (hepatitis) from normal dose |
|
|
isoflurane
|
inhalational general anesthetic
less cardiac depression than with others |
|
|
desflurane
|
inhalational general anesthetic
not used anymore least soluble in blood (fast in/out) |
|
|
sevoflurane
|
inhalational general anesthetic
-smooth induction, less irritating to airways -useful in children, rapid emergence |
|
|
nitrous oxide
|
"laughing gas", a general anesth
Pros: good analgesic, allows ↓ other agents Cons: weak (no induced unconsc), hypoxia, expands air spaces |
|
|
common problems in general anesthesia
|
1. circulatory depression (fluids, vasopressors)
2. resp. depression (airway control, ventilation) 3. awareness (monitor EEGs) |
|
|
malignant hyperthermia
|
*condition of hypermetabolism
-↑ intracel Ca2+, ↑↑temp -b/c of inhal agents and succinylcholine; genetic -↑ mortality; tx: dantrolene |
|
|
propofol
|
intravenous general anesthetic-most common, good for induction and maintenance; wake up from pleasant/sexual dreams
-anti-nausea |
|
|
sodium thiopental
|
barbituate; intravenous general anesthetic
-truth serum |
|
|
ketamine
|
intravenous general anesthetic
-dissociative, phencyclidine (PCP) = hallucinations ↑ BP and HR (only one), releases catecholamines |
|
|
midazolam
|
a benzodiazepine, intravenous general anesthetic
|
|
|
ideal general anesthetic agent
|
= combination of agents
-reduction of each dos -reduction of toxic effects -synergism |
|
|
angina pectoris
|
-sx of ischemic heart disease
-imbalance b/w myocardial oxygen demand (↑) and supply (↓) = severe, sudden chest pain -2 types: typical & variant |
|
|
Typical Angina
|
aka Angina of Effort
-↑O2 demand (exercise, cold, emotion, eating) and ↓ supply (atherosclerotic coronary artery disease) = S-T depression (EKG) |
|
|
Variant Angina
|
cx: vasospasm of coronary artery, w|w/o atherosclerosis
-chest pain develops at rest |
|
|
Therapeutic Aim for Angina
|
*improve balance b/w O2 D and S
-typical: ↑ coronary blood flow, ↓myocardial workload -variant: ↓/prev coronary vasospasm |
|
|
organic nitrates
|
rx for angina = relax vasc. SM
esters of nitric acids -can be short acting (nitroglycerine, GTN) or long acting (ISDN) |
|
|
nitroglycerine
|
short acting organic nitrate
rx for angina pectoris |
|
|
glyceryl trinitrate
|
GTN, short acting organic nitrate
rx for angina pectoris |
|
|
isosorbine dinitrate
|
ISDN, long acting organic nitrate
rx for angina pectoris |
|
|
mechanism of action for organic nitrates
|
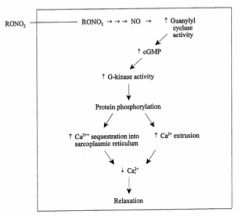
RONO2 = organic nitrate
|
|
|
Decreased Myocardial Oxygen Requirement Action of ON's
|
*venodilation (↓ venous return = ↓ preload = ↓ work = ↓O2 nd)
*arteriolar dilation (↓ periph resist = ↓ afterload = ↓ work) |
|
|
Improved Perfusion of Ischemic Myocardium Action of ON's
|
can dilate vasc to healthy areas to force blood to the blocked area (GTN) or divert towards healthy area (coronary steal, dipyridamole)
|
|
|
Absorption of ON's
|
*sublingual
*oral *skin *IV to heart suring failure/buccal/inhalation (rec) |
|
|
sublingual absorption of ON's
|
-short duration
-nitroglycerine (GTN) main drug (effects for intense) --peak effcts ~3 min, last for 20-30 min |
|
|
oral absorption of ON's
|
common route for ISDN
-extensive first-pass metabolism by glutathione transferase -higher doses than subling. |
|
|
glutathione transferase
|
enzyme responsible for first pass metabolism of ISDN
|
|
|
skin absorption of ON's
|
2% GTN ointment = effective long lasting preparation ~4 hrs
-GTN discs/patches: allow gradual absorption over 24 hours (?tolerance problem) |
|
|
adverse effects of ON's
|
b/c of vasodilation:
*headache (common and severe) *flushing (head, neck) *dizziness & weakness *methemoglobemia |
|
|
methemoglobemia
|
*adverse effect of ON's
-oxidation of heme iron in RBC's by nitrite anion released during metabolism w high infusion rates -tx for cyanide poisoning |
|
|
nitrate tolerance
|
only seen with: *chronic oral ISDN: ↓antianginal and hemodynamic effect;
*transdermal GTN patches |
|
|
prevention and mechanism of ON tolerance
|
prev by having drug-free period
mech: ↓ vasc biotransformation = altered guanyly cyclase = ↑ phosphodiesterase activity |
|
|
therapeutic uses of organic nitrates
|
*termination or preention of individual angina attack (GTN sublingual)
*chronic prophylaxis of angina (ISDN ↓ freq of attacks) |
|
|
Calcium Entry Blockers
|
block L-type Ca2+ channels
-mostly dihyropyrimidine compounds -ex. nifedipine, verapamil, diltiazem |
|
|
mechanism of action of Calcium Entry Blockers
|
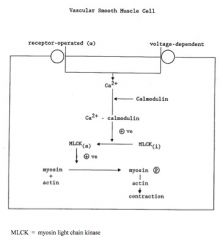
block voltage-dependent calcium channels in vascular smooth muscle and cardiac tissue
|
|
|
Effects of Calcium Entry Blockers on cardiac function
|
*negative inotropic effect
*↓ pacemaker rate at SA node *↓ conduction through AV node |
|
|
Differential Actions of Calcium Entry Blockers (N vs D vs V)
|
Nifedipine blocks channels in vascular smooth muscle at much lower doses (card effects not observed) vs Verapamil and Diltiazem potent cardiac effect
|
|
|
Anti Anginal Effects of Calcium Entry Blockers
|
1. arteriolar dilation
2. Prevent/inhib coronary vasospasm *ideal for variant* 3. Cardiac effects of V and D = ↓ work |
|
|
pharmacokinetics of Calcium Entry Blockers
|
-all well absorbed orally
-bioavail 20-50% b/c first pass -plasma protein binding >90% V and N; half-life 3-5 hours -urine: N & V, feces: D |
|
|
adverse effects of Calcium Entry Blockers
|
1. cardiac depression (V,D) - bradycardia, A-V block, CHF
2.excessive hypotension (N) 3. flushing, edema, dizziness, nausea |
|
|
β-adrenergic antagonists
|
↓ severity/freq of attacks in typical, not useful in variant and may worsen (↑ coronary resistance); ex. propanolol
-negative ino and chronotrope |
|
|
ivabradin
|
new antianginal; blocks pacemaker current in SA node (If channels) = ↓HR w/o iono change
|
|
|
ranolazine
|
inhibits fatty acid oxidation (req. more O2 to generate ATP than glucose) = ↑ gluc utiliz. for ATP prod = ↓ O2 consumption
|
|
|
chemistry of digitalis
|
-cardiac glycoside; unsaturated lactone ring is essential, angle of C and D ring is unique, pham act. resides in aglycone, sugars determine water and lipid sol
|
|
|
sources of digitalis
|
plants: foxglove (D. purpurea) = digitoxin, D. lananta = digitoxin, digoxin, deslanoside;
|
|
|
congestive heart failure: common causes and result
|
cx: coronary artery disease, myocardial infarction, hypertension
= ↓contractility = ↓CO below phys requirements |
|
|
intrinsic compensatory mechanisms of congestive heart failure
|
*pressure-volume relationship: ↓ ejection fraction = ↑ventricular V & P = ↑fibre stretch = ↑contractility
(frank-starling) |
|
|
extrinsic compensatory mechanisms of congestive heart failure
|
*↑sympathetic activity
CV: ↑HR & contractility renal: ↑renin/angiontensin/aldost ↑NaCl/H2O retention, BV |
|
|
therapeutic modalities for congestive heart failure
|
*salt restriction
*diuretics *vasodilators *positive inotropic agents (digitalis) |
|
|
effects of digitalis on congestive heart failure
|
↑CO = ↓compensatory mechanisms
= ↓ heart size, symp act, HR, vasoconstrict, edema, renal perfusion, venous congestion |
|
|
Mechanism of Action of digitalis
|
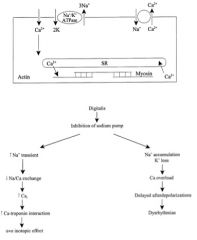
binds and inhibits Na/K ATPase in cardiac tissue leading to an increase in [Ca2+]
|
|
|
pharmacological actions of digitalis
|
mechanical: ↑contractility
electrical direct and indirect effects |
|
|
direct electrical pharmacological effects of digitalis
|
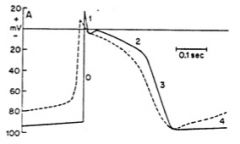
↓RMP = ↓phase 0 slope & cond velocity
↓duration phase 2 = ↓APD & ERP ↑slope phase 4 = ↑ automaticity |
|
|
indirect electrical pharmacological effects of digitalis
|
*↑ vagal act in CNS
*↑ baroreceptor sensitivity *↓ response to NE at SA and AV nodes =↓conduction vel &↑ERP |
|
|
adverse effects of digitalis
|
*GI: anorexia, nausea, vom, dia
*neuro: headeach, fatigue *vision: clurred, dist colour *CV: (elec. eff) sinus brad, A-V block, ventric. dysrhythmia |
|
|
treatment of adverse effects of digitalis
|
*GI/visual: withhold dose
*occ extra beat/bigeminy = oral K+ and drug withdrawal; more serious = IV K+ & antdysrhyth *OD: digitalis antibodies |
|
|
drug interactions with digitalis
|
*K+ competes for Na/K binding
*quinidine: displaces from tissues, double levels, ↓ clear *antibiotics: inhib gut flora from inact dig = ↑ blood levels |
|
|
pharmacokinetics of digoxin
|
absorption: 40-70%
PPB: 25% Peak Time: 3-6 hrs Half-life: 1.6 days therap []: 0.5-2 ng/mL |
|
|
methods of digitalization
|
*rapid: loading + maintenance dose
*just maintenance dose: dep on renal fxn (1.6 days, 35%/day or 4.4 days, 14%/day) |
|
|
RMP
|
resting membrane potential
~= E(K+) |
|
|
Nernst equation
|
EK+ = -61*log[Ki]/[Ko]
|
|
|
pacemaker rate
|
depends on
1. maximum diastolic voltage 2. slope of phase 4 3. threshold voltage |
|
|
ERP
|
effective refractory period
many antidysrhythmics lengthen it |
|
|
responsiveness
|
maximum rate of depolarization in phase 0 (Vmax)
-depends on RMP at moment of depolarization, reflects recovery of Na channels |
|
|
conduction velocity
|
depends on:
1. action potential amplitude 2. slope of phase 0 |
|
|
causes of dysrhymthias
|
ischemia, altered electrolytes, ↑ catecholamines, drugs (digitalis), diseased/scarred tissue = disturbed impulse generation or conduction
|
|
|
automaticity
|
disturbed impulse generation
*altered normal automat = ectopic focus, changes in P4 *abnormal automat: delayed after depolarizations |
|
|
ectopic focus
|
SA node slows down or latent pacemakers speed up
|
|
|
disturbed conductance
|
re-entrant dysrythmias
-need: obstacle to cond, unidirectional block, conduction time through damaged area > in other areas |
|
|
mechanisms of antidysrhythmic drugs
|
*reduce pacemaker activity by ↓ P4 slope
*modify impaired conudction (Na or Ca channel block, β block, ↑ ERP) |
|
|
Class I Na-Channel blockers (incl mechanism and examples)
|
↑affinity for A/I state, ↓ R
ex. quinidine, lidocaine *use-dependent blockade *↓# avail channels, ↑ R recovery time |
|
|
Quinidine (class, etiology)
|
Class IA
1.uni → bidirectional block: A state(↓cond v), I state(↑ ERP), K+block(↑APD) 2.↓ automaticity |
|
|
uses of quinidine
|
-wide spectrum
-ventricular and supraventricular tachydysrhythmias |
|
|
toxicities of quinidine
|
SA, AV block
GI upset Cinchonism (tinnitus, blurred vision, headache) |
|
|
Lidocaine (cardiac)
|
Class IB;short half-life (15 min) = IV; greater effect in ventric and Purkinje cells;
1.↓automaticity 2. ↓ cond v in depolarized tissue only (I>A) |
|
|
uses of lidocaine (cardiac)
|
-ventricular dysrhythmias
-myocardial infarct -open heart surgery -digitalis toxicity |
|
|
Class II Antidysrhythmics
|
β-blockers; ex. propanolol
-opp symp act, esp in AV node =↑ REP, control ventric rate -used for supraventricular dys |
|
|
Class III
|
Amiodarone (lots o toxicities)
-blocks Na & K channels; I state block ↑ ERP, K block ↑ APD -long half-life 13-100 days -prophyl control of vent tachy |
|
|
Class IV
|
Ca-Entry blockers; Verapamil, Diltiazem
= ↓A-V conduction -used for supraventricular dys |
|
|
Class V (Other)
|
Adenosine
↑K conduct = hyperpol membrane = slowing AV conduction -very short 1/2life (secs) -use: paroxysmal surpavent tachy |
|
|
primary/essential hypertension
|
systolic: > 140 (majority, rel to age)
diastolic: > 90 -majority don't have both -no known cause |
|
|
secondary hypertension
|
known cause
ex. renal artery stenosis ex. adrenal tumours (medulla = pheochromocytoma, cortex = hyperaldosteronism) |
|
|
factors controlling blood pressure
|
*cardiac output
*vasocontrictor tone (PVR) *blood volume (kidney) *vascular structure (contractility) |
|
|
regulatory mechanism for cardiac output
|
baroreceptor reflex: ↓stretch = ↓BP = ↑symp outflow = ↑HR and contractility = CO (also vasoconstrict = ↑PVR)
*to maintain steady state |
|
|
regulatory mechanisms for vasoconstrictor tone
|
local: EDRF = NO, EDCF, kinins, ET-1;
neural: sympathetic nervous system humoral (NF, EPI, Ang II, etc) |
|
|
regulatory mechanisms for blood volume
|
↓renal perfusion or ↑symp act
= ↑renin, Ang II, aldosterone = ↑H2O and [salt] *kidney established set-point for long-term arterial P level |
|
|
regulatory mechanisms for vascular structure
|
*structurally-based ↑ in vasc resistance by:
↑wall thickness |
|
|
therapeutic targets for vasocontrictor tone
|
*sympathetic nervous system inhibitors
*vasodilators *??renin-angiotensin system blockers |
|
|
sympatholytics
|
inhib SNS: centrally acting (chlonidine), ganglionic blocking, α/β/mixed-receptor antagonists
|
|
|
vasodilation of constricted blood vessel as rx for hypertension
|
*vasodilators: hydralazine, minoxidil; *Ca channel clockers: verapamil, amlodipine; *K channel activators
-wide var of adverse effects |
|
|
therapeutically targetting blood volum as rx for hypertension
|
1. diuretics
2. act on RAS system |
|
|
targetting RAS system for tx of hypertension
|
*angiotensin convertin enzyme (ACE) inhibitors: ramipril etc
*angiotensin II receptor blockers (ARBs): losartan etc -block AT1's; = fewer adv effect |
|
|
disorders that need diuretics
|
-hyptertension
-heart failure -edema -acute altitude sickness |
|
|
natriuretics
|
increase in urine sodium, are also diuretics because water follows sodium (most important diuretics)
|
|
|
average extracellular fluid
|
12.5 L
|
|
|
average GFR
|
glomerular filtration rate
125 ml/min |
|
|
average urine production
|
1 ml/min
|
|
|
glomerular filtration (and drugs that target)
|
affected by BP and flow
rx: inotropes (tx congestive heart failure), vascular agents |
|
|
osmotic diuretics (prototype, mechanism, requirements)
|
*mannitol
mech: maintains osmotic strength in forming urine req: freely filtered, not reabsorbed, pharm inert |
|
|
osmotic diuretics (adverse effects)
|
-high osmotic load may shift too much fluid into intracellular space = pulm congestion, congestive heart failur
-dose used; 50-200g/day, 25% |
|
|
osmotic diuretics (pharmcokinetics, uses)
|
p: not absorbed from GIT, excreted unchagned in urine
u: prevent renal failure, diuretic, ↓CSF and intraocular pressure |
|
|
thiazides (prototype, mechanism)
|
*hydrochlorothiazide
m: ↓NaCl reabsorp at luminal surface = ↑ water excretion |
|
|
thiazides (pharmakokinetics)
|
-absorbed orally
-actively transported into lumen, proximal convoluted tubule -uric acid retention |
|
|
thiazides (adverse effects)
|
*hypokalemia
-↓carbohydrate tolerance -hyponatremia -↑lipids, LDL |
|
|
loop/high ceiling diuretics (prototype, action)
|
*furosemide
-acts on ascending limb -↓ Na/K/Cl transport -potency weak at low doses, but higher limit |
|
|
loop diuretics (adverse effects)
|
-mainly hyopkalemia
-uric acid retention -excessive fluid and electrolyte loss |
|
|
carbonic anhydrase inhibitor (prototype, mechanism)
|
*acetazolamide
-blocks bicarbonate conversion = retention in urine -must have 90% of CA inhibited to produce effect |
|
|
diuretic combinations
|
furosemide + HCT: may be supra-additive
HCT+amiloride: for hypokalemia, safest treatment is K+ supplementation |
|
|
potassium sparing diuretics
|
*spironolactone and amiloride
-both may induce hypokalemia |
|
|
amiloride
|
inhibits K+ excretion
|
|
|
spironolactone
|
competitive aldosterone antagonist
-slow action, most useful in aldosterone excess conditions |
|
|
acetazolamide action and adverse effects
|
-metabolic acidosis (tx alkalosis); effect wanes
-tx for acute altitud sickness (die of pulmonary and cerebral edema); AE: paresthesia, drows |

