![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
Q: Who proposed the first theory on the characteristics of individual atoms and particles?
|
John Dalton
|
|
|
Q: Who blasted particles through gold foil to discover that atoms had positive centers?
|
Ernest Rutherford
|
|
|
Q: Who a cathode ray tube in his experiments and developed the plum pudding model?
|
J.J. Thomson
|
|
|
Q: Which Greek philosopher called tiny particles atomos (atoms)?
|
Democritus
|
|
|
a dense positive core surrounded by mostly empty space
|
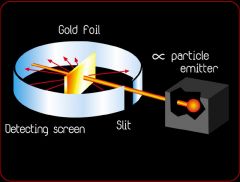
What did this experiment suggest about the atom's structure?
|
|
|
In Thomson's CRT experiments
|
he found that no matter what metal he used or gas he used that.... he got the same result. He deduced that electrons are found in all elements.
|
|
|
Thomson's discoveries led to the formation of the _______ model of the atom
|
with electrons stuck all over a blob of positive plum pudding
|
|
|
Rutherford's model of the atom shows electrons orbiting the nucleus
|
which is why some people call it the ___ model planetary atomic model
|

