![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
130 Cards in this Set
- Front
- Back
|
Define normocytic anemia. |
Anemia with normal-sized RBCs. MCV is 80-100.
|
|
|
The key differential diagnosis in most normocytic anemias is [...]
|
Anemia due to increased peripheral destruction or underproduction. |
|
|
How can you differentiate between normocytic anemia due to increased peripheral destruction or underproduction?
|
Reticulocyte count helps distinguish between these two etiologies.
|
|
|
What are the strands in the cytoplasm of a reticulocyte?
|
Thread-like RNA filaments (gives the cell its blue hue on smear and stain)
|
|
|
Normal reticulocyte count in the blood is __ to __ %.
|
1;2. Each day, 1-2% of cells are removed and replaced by reticulocytes.
|
|
|
RBC lifespan is _________.
|
~120 days
|
|
|
If a healthy man acquires a normocytic anemia, how should the marrow respond?
|
A properly functioning marrow should respond to anemia by increasing RC to > 3%.
|
|
|
Percentage of RCs in anemia is ______________. Why?
|
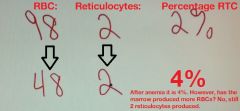
falsely elevated
A decrease in total RBCs, falsely elevates the percentage of reticulocytes. |
|
|
How do we correct a reticulocyte count for the degree of anemia?
|
Correct it by multiplying reticulocyte count by Hct/45
|
|
|
What can the corrected reticulocyte count be used for?
|
To figure out what the etiology of RBC destruction is in normocytic anemia, i.e. are the RBCs being produced sufficiently or being destroyed outside the marrow. A corrected ret count > 3% indicates good marrow function, telling us it is being destroyed.
|
|
|
A corrected reticulocyte count of > ____% indicates good marrow response, while < ___ % suggests underproduction.
|
3;3
|
|
|
Hemolysis can be divided into?
|
(1) Intravascular
- Within blood vessels (2) Extravascular - Destroyed by reticuloendothelial system (macrophages of spleen, liver, lymph nodes) |
|
|
A normocytic anemia with hemolysis would result in a _________ (poor/good) marrow response.
|
good, greater than 3% corrected reticulocyte count.
|
|
|
Macrophages can consume _______ and break down the _______ (pigment).
|
RBCs; hemoglobin
|
|
|
Describe the breakdown of hemoglobin.
|
Hemoglobin = Globin + Heme (iron + protoporphyrin)
Globin - amino acids Heme - iron, protoporphyrin Protoporphyrin - unconjugated bilirubin (indirect) |
|
|
After RBCs are broken down, macrophages spits out unconjugated bilirubin and it becomes bound to ____________. It is then delivered to the _________ for ____________ reactions and excreted in the ________.
|
serum albumin; liver; conjugation; bile
|
|
|
How would patients with extravascular hemolysis present? What are lab findings?
|
(1) Anemia with splenomegaly (hypertrophy)
(2) Jaundice due to unconjugated bilirubin (more than capacity of liver to conjugate) (3) Bilirubin gallstone risk (4) Marrow hyperplasia with corrected reticulocyte count of > 3%. |
|
|
What is the initial phase in intravascular hemolysis of RBCs?
|
Hemoglobin is released into the blood, subsequently complexing with haptoglobin. This is phagocytosed and degraded by macrophages. We see decrease in haptoglobin levels in intravascular hemolysis related anemias.
|
|
|
After haptoglobin salvages some Hb, what is the next step in the evolution of an intravascular hemolysis?
|
Haptoglobin is minimal, so after it is depleted, Hb builds up in blood, creating a hemoglobinemia. Reaches the urine, creating a hemoglobinuria. Some tubule cells absorb Hb, storing iron as hemosiderin. When tubule cells turn over, they slough off and this can be measured in urine as hemosiderinuria.
|
|
|
What is hereditary spherocytosis? Be general, not specific.
|
Inherited defect of RBC cytoskeleton-membrane tethering proteins.
|
|
|
Hereditary spherocytosis most commonly involves these proteins.
|
Spectrin, ankyrin, band 3.1
|
|
|
Describe the pathophysiology of hereditary spherocytosis.
|
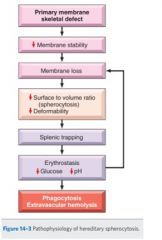
Remember, because of the inherited defect, small blebs of cytoplasm falls off the cell, causing it to round off.
|
|
|
Describe the makeup of parvovirus.
|
Linear ssDNA. Naked icosahedral.
|
|
|
Life span of spherocytes is _____ to _____ days.
|
10;20
|
|
|
How would spherocytes look like on smear?
|
Loss of central pallor. May see a higher than normal RDW due to some cells being older and having lost more membrane.
|
|
|
Spherocytes are less able to maneuver through the _________. What happens?
|
splenic sinusoids
They are consumed by splenic macrophages. |
|
|
What are some clinical and lab findings in HS?
|
(1) Spherocytes with loss of central pallor
(2) Increased RDW (3) Increased MCHC (cell shrinks, Hb is more concentrated) (4) Splenomegaly (macrophages hypertrophy), jaundice with indirect bilirubin, increased risk for gallstones (5) Increased risk for aplastic crisis with parvovirus B19 infection of erythroid precursors. |
|
|
Diagnosis of HS occurs by?
|
Osmotic fragility test (increased fragility in hypotonic solutions)
|
|
|
Treatment of HS? What happens after this?
|
Splenectomy.
(1) Anemia resolves (2) Spherocytes PERSIST (3) Howell-Jolly bodies emerge on blood smear |
|
|
What is a Holly-Jowell body?
|
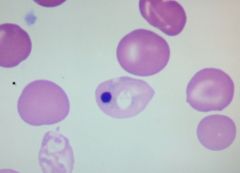
On occasion there might be a RBC where some nuclear material wasn't removed. It's the job of the spleen to remove it.
|
|
|
In sickle cell anemia there is predominantly _______________ (intravascular/extravascular) hemolysis.
|
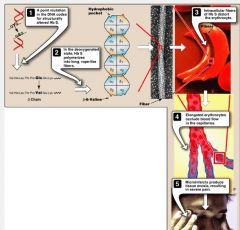
extravascular
|
|
|
In sickle cell anemia, patients have an ____ mutation in the _____ chain gene of hemoglobin.
|
AR; beta
|
|
|
What amino acids are replaced in sickle cell disease?
|
Normal glutamic acid (hydrophilic) is replaced with valine (hydrophobic).
|
|
|
The gene for sickle cell disease is carried by ____% of what ethnic group? What evolutionary significance does this have?
|
10%; African americans
Likely due to protective role against falciparum malaria |
|
|
In intravascular hemolysis there is a risk for becoming _____ deficient.
|
iron
|
|
|
Sickle cell arises when ____ abnormal ______ (beta/alpha) genes are present.
|
2; beta
|
|
|
Sickle cell disease results in > _____% of Hb_ in RBCs.
|
>90%; HbS
|
|
|
What is the primary hemoglobin produced in patients with sickle cell disease?
|
HbS (alpha2beta2[S])
|
|
|
What's the issue with hemoglobin S?
|
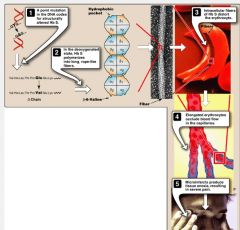
It polymerizes when deoxygenated. Polymers aggregate into needle-like structures resulting in sickle cells.
|
|
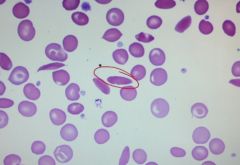
What is this banan shaped cell?
|
Sickle cell
|
|
|
In sickle cell disease, there is an increased risk of sickling with these factors:
|
(1) Hypoxemia
(2) Dehydration (3) Acidosis |
|
|
This hemoglobin is protective against sickling.
|
HbF
|
|
|
Why don't patients with sickle cell anemia present until about 6 months of birth?
|
High HbF at birth is protective for the first months of life.
|
|
|
Treatment of sickle cell disease?
|
Hydroxyurea increases HbF (we don't know why), this helps for sickle cell disease.
|
|
|
Cells in sickle cell disease continuously ________ and ________ while passing through microcirculation.
|
sickle; desickle
|
|
|
Sickle cells passing through microcirculation leads to complications related to ________________. What happens?
|
RBC damage.
(1) Less flexible over time, spleen removes them (extravascular hemolysis). Anemia. (2) Jaundice with unconjugated hyperbilirubinemia. (3) Increased risk for gallstones |
|
|
Is sickle cell anemia extravascular or intravascular mechanism of hemolysis? What is the result of intravascular hemolysis.
|
Both, but extra is predominant.
(1) Intravascular happens and decreases haptoglobin. (2) Cells dehydrate as they are damaged. Cytoplasm is going to be slowly decreased. Creating target cells. |
|
|
What is a consequence of extravascular and intravascular hemolysis in sickle cell disease?
|
Massive erythroid hyperplasia.
(1) Expansion of hematopoiesis into skull and facial bones (2) Extramedullary hematopoiesis with hepatomegaly (3) Risk of aplastic crisis with parvovirus B19 |
|
|
Outside of anemia as a major complication of sickle cell anemia, what is the second major complication?
|
Irreversible sickling. Leads to vaso-occlusion.
|
|
|
Give an example of vaso-occlusion in sickle cell disease. What is it? Who does it present in?
|
Dactylitis.
Swollen hands and feet due to vaso-occlusive infarcts of bones. Common presenting sign in infants. |
|
|
What is this?
|
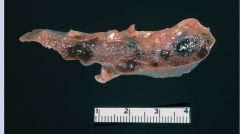
Autoinfarcted spleen in sickle cell disease
|
|
|
What happens to the spleen in sickle cell anemia?
|
It gets repeatedly infarcted, and eventually leading to a shrunken fibrotic spleen.
|
|
|
If the spleen is lost, the primary source of what is lost? This creates an opportunity for infection with ____________.
|
primary source of antibodies is lost.
Encapsulated organisms |
|
|
This is the MC cause of death in children with sickle cell disease.
|
Infection with encapsulated organisms
|
|
|
Sickle cell disease increases the risk for osteomyelitis with this organism.
|
Salmonella paratyphi
|
|
|
Eventually, in sickle cell anemia, the RBCs show _________ bodies.
|
Howell-Jolly
|
|
|
What is acute chest syndrome? How does it present?
|
In the context of sickle cell anemia, there is vaso-occlusion in pulmonary microvasculature.
Presents with chest pain, dyspnea, lung infiltrates. |
|
|
Acute chest syndrome in sickle cell disease is often precipitated by ____________. Why?
|
pneumonia
Infection, inflammation, vasodilation, increased transit time, increased sickling. |
|
|
MCC of death in adults with sickle cell anemia.
|
Acute chest syndrome
|
|
|
Vaso-occlusion in sickle cell anemia can lead to _________ crisis.
|
Pain crisis
|
|
|
A vaso-occlusive crisis in the kidney in the context of sickle cell disease may lead to? This results in?
|
Renal papillary necrosis. Results in gross hematuria and proteinuria.
|
|
|
You need about ____% of HbS for it to start to sickle.
|
50%
|
|
|
Sickle cell trait results in < _____% HbS in RBCs.
|
50%
|
|
|
Patients with sickle cell trait are generally ______________. There is one exception however, what is it?
|
Asymptomatic
Renal medulla (they tend to sickle here). In the medulla there is extreme hypoxia and hypertonicity (mimicking sickling) |
|
|
A patient with sickle cell trait has sickling in her kidneys. What happens there? What does it lead to?
|
Hypoxic and hypertronic environment sickles cells. Causes microinfarctions. Initially presents with microscopic hematuria, and, eventually, decreased ability to concentrate urine.
|
|
|
True or false: Sickle cells and target cells are seen on smear in sickle cell trait.
|
False. They are seen in sickle cell disease, but not the trait.
|
|
|
What is a screening test for sickle cell anemia? Is it positive in trait or disease?
|
Metabisulfite screen, induces cell to sickle. It is positive in both.
|
|
|
How could you confirm the presence of HbS?
|
Hb electrophoresis
|
|
|
What are the relative percentages of different hemoglobins in sickle cell disease?
|
(1) 90% HbS
(2) 8% HbF (3) 2% HbA2 (no HbA; no beta chains) |
|
|
What are the relative percentages of different hemoglobins in sickle cell trait?
|
(1) 55% HbA
(2) 43% HbS (3) 2% HbA2 |
|
|
What is Hemoglobin C?
|
A hemoglobinopathy. AR mutation in beta chain of hemoglobin. Normal glutamic acid is replaced by lysine.
"Hemoglobin C has lyCIIIIIIIIINE" |
|
|
Hemoglobin C is __________ (more/less) common than sickle cell disease.
|
less
|
|
|
How does Hemoglobin C present?
|
Mild anemia due to extravascular hemolysis.
|
|
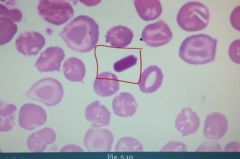
What is this?
|
Characteristic HbC crystals (lysine and hemoglobin C crystals) are seen in RBCs of patients with Hemoglobin C.
|
|
|
What is paroxysmal nocturnal hemoglobinuria?
|
Intravascular hemolysis. ACQUIRED defect in myeloid stem cells resulting in absent GPI. Renders cells susceptible to complement (which is more active in more acidic conditions, as when you sleep).
|
|
|
What is the molecule(s) used by RBCs (and other cells) to protect themselves against their deadly neighbour?
|
(1) Decay accelerating factor (DAF)
(2) MIRL - Membrane inhibitor of reactive lysis |
|
|
DAF accelerates the degradation of ___________________.
|
C3 convertase
|
|
|
DAF and MIRL are both connected to the RBC membrane by a protein called a __________.
|
GPI (Anchoring protein)
|
|
|
What cell types can become lysed in PNH?
|
(1) RBCs
(2) WBCs (3) PLTs |
|
|
Why does PNH occur during the night?
|
When we sleep we breathe shallow. Mild respiratory acidosis ensues, activating complement more readily.
|
|
|
What laboratory indices are seen with PNH?
|
(1) Hemoglobinemia
(2) Hemoglobinuria (3) Hemosiderinuria is seen days after hemolysis |
|
|
How do you screen for PNH?
|
Sucrose test is used to screen for disease. It activates complement in the blood.
|
|
|
What is a confirmatory test of PNH?
|
Confirmatory test is acidified serum or flow cytometry to detect lack of CD55 (DAF).
|
|
|
DAF is also known as cluster of differentiation ____.
|
55 (CD55)
|
|
|
What is the main cause of death in patients with PNH?
|
Thrombosis of hepatic, portal or cerebral veins. Since PLTs are destroyed, cytoplasmic contents are released into circulation inducing thrombosis.
|
|
|
Complications of PNH include?
|
(1) Iron deficiency anemia
- Hemoglobinuria (2) AML (develops in ~10% of patients) - Remember, this dx is a mutation in myeloid stem cell. |
|
|
G6PD is a predominantly _____________ (intravascular/extravascular) disorder.
|
intravascular
|
|
|
What is the inheritance pattern of G6PD? What happens to the enzyme?
|
X-linked recessive
It has a reduced half life |
|
|
G6PD renders cells susceptible to what?
|
Oxidative stress
|
|
|
RBCs live in an environment of ___________.
|
oxidative stress
|
|
|
Why are RBCs more susceptible to oxidative stress in G6PD deficiency?
|
G6PD participates in making NADPH, needed to reduce GS-SG back to GSH such that it can neutralize H2O2.
|
|
|
G6PD has two major variants. What are they?
|
(1) African-variant with mildly reduced half life
(2) Mediterranean variant with markedly reduced half life |
|
|
Why is there a high frequency of G6PD in african americans and people of mediterranean descent?
|
Likely due to protective role against falciparum malaria.
|
|
|
Causes of oxidative stress to RBCs include?
|
(1) Infections
(2) Drugs (primaquine, sulfa drugs, dapsone) (3) Fava beans |
|
|
When hemoglobin hits an oxidative stress, what happens?
|
It causes Hb to precipitate as a Heinz body. It is removed by splenic macrophages, resulting in bite cells, leading to intravascular hemolysis.
|
|
|
How does G6PD deficiency present?
|
Hemoglobinuria and back pain (hemoglobin is nephrotoxic). Occurs hours after exposure to an oxidative stress.
|
|
|
If you want to see the precipitated hemoglobin (Heinz), you won't be able to see it on a smear. You must do something first, what is it?
|
Heinz preparation. Used to screen for disease.
|
|
|
How do you confirm G6PD disease after screening?
|
Confirm it by enzymatic studies.
|
|
|
A patient with G6PD has an acute hemolytic episode. You want to confirm this disease by doing enzyme studies. It comes back negative. Why might it be falsely negative?
|
You have to perform the test in between hemolytic episodes. All cells present in the blood sample will lack the enzyme (others being lysed).
|
|
|
Immune hemolytic anemia is an ____________mediated destruction of RBCs.
|
Antibody (IgG or IgM)
|
|
|
IgG-mediated disease usually involves __________________ (intravascular/extravascular) disease.
|
extravascular
|
|
|
IgG in the context of immune hemolytic anemia is also called _________________.
|
warm agglutinin because it binds RBCs in the relatively warm central body
|
|
|
What is the resulting shape of RBCs in IgG-mediated hemolytic disease?
|
Spherocytes. Membrane of antibody-coated RBCs is consumed by splenic macrophages.
|
|
|
IgG-mediated hemolytic disease is associated with what?
|
(1) SLE
(2) CLL (neoplastic B-cells here do not have a desire to do anything beneficial, not only do they not produce Abs, but when they do so, they do a poor job) (3) Certain drugs (like penicillin, which could adhere to RBC membrane which removes pieces of membrane). The drug could also induce production of Ab itself (methyldopa). |
|
|
Treatment of IgG-mediated hemolysis?
|
(1) Remove offending drug
(2) Steroids (slow down production of Ab) (3) IV Ig (splenic macrophages eat IgG instead; or Fc receptors are blocked) (4) Splenectomy |
|
|
IgM-mediated hemolysis usually involves ________________ (intravascular/extravascular) hemolysis.
|
intravascular
|
|
|
In IgM-mediated hemolysis, IgM binds and fixes complement on RBCs where?
|
Usually in the colder temperature of the extremities (called cold agglutinins).
|
|
|
IgM-mediated hemolysis is associated with what other diseases or infections?
|
(1) Mycoplasma pneumoniae
(2) Infectious mononucleosis |
|
|
What is used to diagnose immune hemolytic anemias?
|
Coombs test. Can be direct or indirect.
|
|
|
What is the "Question" behind the use of Direct Coombs test. How does it work?
|
"Do I have RBC bound to IgG".
Anti-IgG is added to patients RBCs. Agglutination occurs if patients RBCs are already coated by IgG. |
|
|
What is the most important test for IHA?
|
Coombs
|
|
|
What is the "Question" behind the use of indirect Coombs test. How does it work?
|
"Does the patient have antibodies in their serum?"
You take test RBCs, put it in the patients serum, add anti-IgG. Agglutination occurs if there is antibodies. |
|
|
What is the HELLP syndrome?
|
HELLP syndrome
(1) Hemolytic anemia with schistocytes (2) ELevated serum transaminases (3) Low Platelets • Due to disseminated intravascular coagulation |
|
|
Microangiopathic hemolytic anemia can occur with what conditions of the heart?
|
(1) Prosthetic heart valves
(2) Calcific aortic stenosis |
|
|
Microangiopathic hemolytic anemia can occur with conditions that produce microthrombi. What are they?
|
(1) TTP-HUS
(2) DIC (3) HELLP |
|
|
Microthrombo produce ___________ on blood smear.
|
schistocytes
|
|
|
Malaria results in infection of __________ and _________ with __________ (species).
|
RBCs; liver; plasmodium
|
|
|
Malaria is transmitted by ________________.
|
female Anopheles mosquito (moskito, mygg)
|
|
|
Regarding malaria. RBCs rupture as part of what?
|
Plasmodium life cycle.
|
|
|
Each time Plasmodium undergoes its life cycle in RBCs, what happens? Describe fever patterns in each spp.
|
Results in intravascular hemolysis and cyclical fever
P. falciparum - daily fever P. vivax, P.ovale - fever every other day |
|
|
Where else than intravascularly does hemolysis occur in malaria? What is the result?
|
Spleen. It consumes some infected RBCs. Results in mild extravascular hemolysis with splenomegaly.
|
|
|
What type of anemia is characterized by a low corrected reticulocyte count?
|
Anemias due to underproduction. It can't compensate.
|
|
|
What are the etiologies of anemia due to underproduction?
|
(1) Causes of microcytic and macrocytic anemias
(2) Renal failure (less EPO produced) (3) Dmg to bone marrow precursor cells - Parvovirus B19 |
|
|
What is aplastic anemia?
|
When you have damage to hematopoietic stem cells. Results in a pancytopenia with low reticulocyte count.
|
|
|
What are etiologies of aplastic anemia?
|
(1) Drugs or chemicals
(2) Viral infections (3) Autoimmune damage |
|
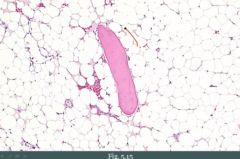
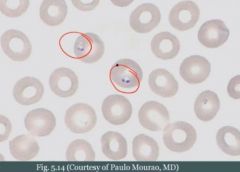
What do you see?
|
A spicule of bone, and fat. This is an empty marrow. Can be seen in aplastic anemia.
|
|
|
Treatment of aplastic anemia involves?
|
(1) Cessation of any drug that may be involved
(2) Transfusions to support the patient (3) Marrow-stimulating factors (EPO, GM-CSF, G-CSF) (4) Immunosuppression (remember, one etiology is autoimmune) (5) Bone marrow transplant is last resort |
|
|
What is myelophtisic process?
|
A pathologic process that replaces the bone marrow. Hematopoiesis is impaired, resulting in pancytopenia. E.g. metastatic cancer.
|
|
|
What cancers most often cause a myelophtisic process?
|
Breast, lung, prostate
|
|
|
What shape of RBCs would you expect on a smear in myelophtisic process?
|
Tear-drop shaped RBCs called dacrocytes.
Believed to be deformed during their tortuous escape from the fibrotic marrow. |

