![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
64 Cards in this Set
- Front
- Back
|
define labile cells
|
cells that are continuosly dividing (hematopoietic ,surface epithelia)
|
|
|
definte stable cells
|
aka quiescent, minimally replicative, proliferate in responses to injury (parenchyma of solid organs, endothelial cells, fibroblasts, smooth muscle cells)
|
|
|
define permanent tissues
|
non-proliferative (neurons, cardiac muscle cells)
|
|
|
define hypertrophy. In which type of cells does it take place?
|
increase in the size of cells by increaseing the amount of proteins and organelles. Occurs in cells that have limited or no capacity to divide
|
|
|
Given an example of physiologic hypertorphy
|
an increase in functional demand or homonal stimulation leads to an increase in cell/ organ size-weight lifting=bigger muscle
|
|
|
Give an example of pathologic hypertrophy
|
cardiac muscle hypertrophy in response to hypertension
|
|
|
Define hyperplasia. In which type of cells does it occur?
|
increase in cell number, occurs in cells capable of division (labile and stable)
|
|
|
Give an example of physiologic hyperplasia
|
hormonal hyperplasia of female breast at puberty/ pregnancy, compensatory hyperplasia of liver after partial resection
|
|
|
Give an example of pathologic hyperplasia
|
excessive stimulation by growth factors or homones, imbalance=endometrial hyperplasia, release of GF's due to HPV=squamous hyperplasia
|
|
|
define atrophy
|
decrease in cells size and function due to loss of cells substance, decrease in protein synthesis and increase degradation, there is a loss of fxn but no cell death
|
|
|
give an example of physiologic atrophy
|
loss of hormonal stimulation -endometrium @ menopause
|
|
|
give some examples of pathologic atrophy
|
broken arm=decreased functional deamand, loss of innervation, inadequate nutrition
|
|
|
define metaplasia
|
one adult cell type is replaced by another adult cell type. adaptive response to chronic stress/ injury
|
|
|
Give an example of epithelial and mesenchymal metaplasia
|
epithelial-in smoker lung ciliated=> squamous,in barrett esophagous squamous=> glandular; mesenchymal-bone formation in soft tissue
|
|
|
what are the two key features of irreversible cell death
|
1. inability to reverse mitochondrial dysfunction (lack of ox phos and ATP generation) 2. disturbance of membrane fxn
(explain death at M&M) |
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: cell size
|
necrosis-enlarged,
apoptosis-reduced, reversible- enlarged (hydropic or vacuolar) |
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: nucleus
|
necrosis-pyknosis, karyorrhexis, karyoLYSIS;
apoptosis-fragmentation into nucelosome size fragments; reversible-clumping of chromatin |
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: plasma membrane
|
necrosis-disrupted;
apoptosis-intact but altered structure; reversible-swollen but intact |
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: cellular contents
|
necrosis-enzymatic digestion, may leak; apoptosis-intact but released in apoptotic bodies, eosinophilia, blebs; reversible-vacuoles distend ER, lipid vacuoles in cytoplasm
|
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: adjacent inflammation
|
necrosis-frequent; apoptosis-no; reversible-maybe
|
|
|
Compare necrosis, apoptosis, and reversible injury in terms of: phys vs. path role
|
necrosis-always path; apoptosis-often phys, can be path; reversible-path
|
|
|
What is the difference between hypoxia and ischemia? Which is "worse"?
|
Hypoxia=inadequate oxygenation of the blood, ischemia=lack of blood. Ischemia is worse because not only does the tissue lack oxygen, it also lacks nutrients required to continue anaerobic metabolism
|
|
|
Cellular swelling and fatty change are characteristics of the morphology of reversible cell injury. WHat causes these features?
|
Cellular swelling resutls from failure of membrane pumps to maintain homeostasis, vacuoles appera in the cells corresponding to a distended ER. Fatty change refers to lipid vacuoles in the cytoplasm of cells dependent on fat metabolism, occurs a result of toxic/ hypoxic injury
|
|
|
Describe the morphological features of necrosis
|
increased eosinophillia (result of increased binding of eosin to denatured cytoplasmic protiens and loss of basophilia that is normallly imparted by RNA in the cytoplasm), nuclear shrinkage and fragmentation, breakdown of plasma and organelle membranes
|
|
|
What is the cause of coagulative necrosis
|
hypoxic or anoxic injury due to ischemia in solid organs (except brain)
|
|
|
Describe the morphology of coagulative necrosis
|
persistence of dead cells with intact outlines but with loss of cellular details (ghosts), injury denatures proteins and enyzymes but no proteolysis takes place, contrast to liqeufactive and caseous necrossis where there is a loss of tissue architecture
|
|
|
What is the cause of liquefactive necrosis
|
bacterial or fungal infection, infarct in the brain
|
|
|
Describe the features of liquefactive necrosis
|
release of digestive enzymes from WBCs leads to complete digestion of the cells, acute inflammation and pus (note that in the brain, acute inflammation is usually not present the tissue is soft and soupy but there is no pus)
|
|
|
What is the cause of caseous necrosis
|
tuberculous infection
|
|
|
describe the features of caseous necrosis
|
resemebles cheese, fragmented and coagulated cells with loss of tissue architecture (no cell outlines), usually surronded by a boarder of inflammatory cells forming a granuloma
|
|
|
Describe gangrenous necrosis
|
a specific type of ischemic coagulative necrosis of the extremity. can be dry or wet (bacterial infection), also used for severe necrosis of other organs
|
|
|
What is the cause of fat necrosis? Where does it occur?
|
Typically seen in the pancrease in acute pancreatitis when injury to the pancreases releases lipases which liquefies fat and splits TGs The FA's combine with Ca to form chalky white substance. Can also occur as a result of trauma to fatty itssue (breast)
|
|
|
What is the cause of fibrinoid necrosis
|
deposition of immmune complexes (antigens+ antibody) in the vascular wall. Occurs in vasculitis syndromes
|
|
|
Describe the features of fibrinoid necrosis
|
"fibrin-like", bright pink amorphous appearance in vascular wall
|
|
|
Where does fibrinoid necrosis occur?
|
vascular wall as a result of deposition of immune complexes
|
|
|
What is lipofusin? Where is it found?
|
indigestible material resulting from lipid peroidation, a "wear and tear" pigment th eoccurs predominately with aging found esp. in heart, liver, brain
|
|
|
Describe anthracosis in the lung
|
carbon particles inhaled and phagocytosed by alveolar macrophages, transported to region lymph nodes
|
|
|
Hypertrophy of the smooth ER in the liver can be a result of..
|
adpative response to increased demand for toxin removal i.e. alcohol, barbiturates
|
|
|
"parking lot inclusions" are a sign of damage to which organelle
|
mitochonria. changes include atrophy (starvation), enlargement (toxin, alcohol), myopathy (respiratory chain enzyme abnormality)
|
|
|
Give two examples of change that can occur to the cytoskeleton as a result of cell injury
|
1. accumulations-toxins like alcohol-Mallory hyaline liver, Alzheimer's and NF tangles
2. abnormal organization-Kartagener Syndrome-immotile cilia = sterility and lung infections |
|
|
List 4 specific targets of cellular injury
|
1. mitochondria 2. calclium homeostasis 3. cellular membrane 4. DNA & cellular protiens
|
|
|
What are the major causes of ATP depletion in a damaged mitochondria
|
decreased oxygen, decresed nutrients, specific toxins
|
|
|
Describe the effects of incrased intracellular calcium that occurs with cell injury
|
too high of intracellular Ca activates cell enzyems which leads to breakdown of phospholipids, disruption of the membrane and cytoskeletal proteins, and nuclear damage. Additionally, the high Ca increases mitochondrial permeabiliyt which decreases the avaiability of ATP.
|
|
|
Explain how cell damage can lead to accumulation of ROS and oxidative damage
|
Cell injury leads to production of ROS in the mitochondria which can cause lipid peroxidation= membrane damage, protein modifications= breakdown and misfolding, and DNA damage= mutations
|
|
|
Describe the mechanism of reperfusion injury
|
restoration of blood flow to ischemic tissue may increase injury because the new oxygen allows for generation of free radicals. Additionally the arriving leukocytes, plasma proteins, and complement can lead to inflammation and the proudction of ahdesion molecuels and cytokines that attract more inflammatory cells that increase the extend of the injury
|
|
|
What is the difference between a direct toxin and a toxic metabolite
|
direct toxin-bind to cellular organelle or molecular component, no modification is necessary for toxic effect; toxic metabolite-non toxic substance is "activated", often by P450 in the liver
|
|
|
List three key morphologic features of apoptosis
|
1. eosinophilic cytoplasm 2. cell shrinkage 3. condensed nucelus
|
|
|
What are the two ways in which apoptosis can be initiated. What is the common end pathway
|
1. intrinsic pathway-cell injury leads to activation of the Bax proteins which increase the perm. of the mitocondria and allow cyt C to enter the cytoplasm.
2. extrinsic pathway-death receptor is engaged (Fas, TNF), common feature is the activation of capases |
|
|
How do dystrophic and metastatic calcification differ in terms of tissues invovled?
|
distrophic-non-viable, damaged or dying tissues. metastatic-normal tissues
|
|
|
How does the serum calcium level differ in dystrophic vs. metastatic calcification
|
dystrophic-normal serum calcium
metastatic- hypercalcemia (high PTH, destruction of bone, D intox, renal failure) (Hint: Metastatic "forces" Ca into normal tissue so need excess Ca) |
|
|
Describe the morphology of dystrophic calcification
|
gross=white, gritty deposites, microscopically=basophilic
|
|
|
Describe the changes that occur with aging that lead to decreased cellular replication and defective protein homeostasis
|
progressive shortening of telomers and lack of telomerase=progressive replicative senescence
|
|
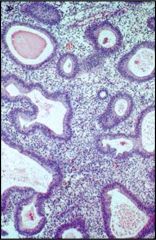
This is an example of endometrial hyperplasia, not ehte increased number of cells and the convolution of the glands
|
-
|
|
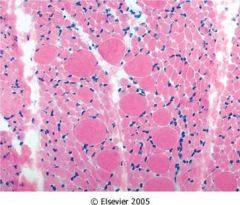
This is skeletal muscle atrophy with compensatory hypertrophy
|
-
|
|
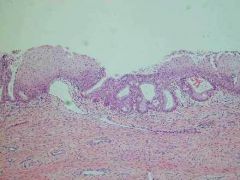
An example of metaplasia of the endocervix, note the transition from normal columnar epithelial to squamous
|
-
|
|
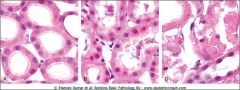
A kidney tubule showing blebs, swelling and eosinophilia of vacular cahnge, transitioning to necrosis. Note the absence of nuclei
|
=
|
|
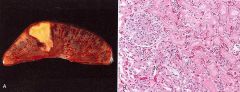
What type of necrosis is shown on the right
|
coagulative
Note the persistence of dead cells with intact outlines but loss of cellular details |
|
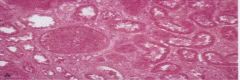
What type of necrosis is this
|
coagulative
|
|
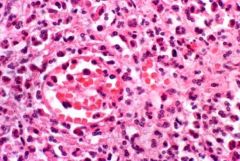
What type of necrosis is this
|
liquefactive
|
|
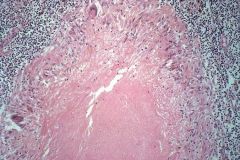
What type of necrosis
|
caseous
|
|
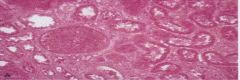
What type of necrosis
|
coagulative
|
|
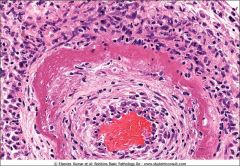
What type of necrosis
|
fibrinoid
|
|
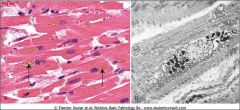
Lipofuscin pigments in cardiac muscle, indeigestible material resuing from lipid peroxidation, a "wear and tear" pigment, occurs predominatly with aging particualry in heart, liver, brain
|
-
|
|
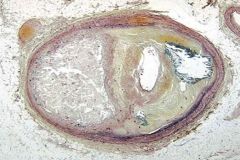
dystrophic calcification within an atherosclerotic plaque
(occurs in damged tissues but normal serum calcium levels) Dystrophic=Damaged |
-
|

