![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Nomenclature of Alcohols
|

Alcohols are slightly acidic (pKa ~ 16).
Alcohol oxygen atoms behave as Lewis bases. Alcohols can react as either bases or nucleophiles at the oxygen. The -OH group is a poor leaving group and needs to be converted to a better leaving group before substitution can occur. Removal of the proton generates the alkoxide. |
|
|
Halogenated Compounds
Functional group suffix = halide (i.e. fluoride, chloride, bromide, iodide) |
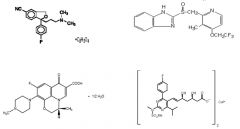
|
|
|
Diols
HOCH2CH2OH = 1,2-ethanediol Functional group prefix = dihydroxy- |
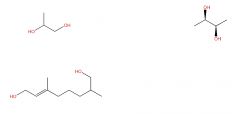
|
|
|
Phenol
|
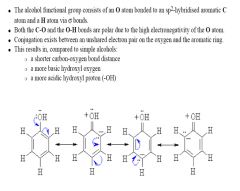
|
|
|
Ethers
Functional group suffix = ether Functional group prefix = alkoxy- Physical Properties: The polar nature of the C-O bond (due to the electronegativity difference of the atoms ) results in intermolecular dipole-dipole interactions. An ether cannot form hydrogen bonds with other ether molecules since there is no H to be donated (no -OH group) Ethers can be involved in H-bonding with systems able to donate H (e.g. water). The implications of these effects are: **lower melting and boiling points compared to analogous alcohols solubility in aqueous media generally slightly less to analogous alcohols. |
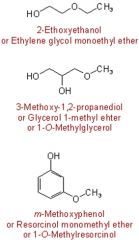
|
|
|
Epoxides
Epoxides are just a sub-class of ethers, in that they contain the C-O-C unit. However, the term "epoxide" is used to describe the 3 membered heterocyclic system. |
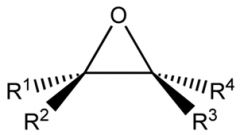
|
|
|
Aldehydes and Ketones
Nomenclature: Aldehydes: functional group suffix = -al Ketones: functional group suffix = -one Physical Properties: The polar nature of the C=O (due to the electronegativity difference of the atoms) means dipole-dipole interactions will occur. Though C=O can not hydrogen-bond to each other, the C=O can accept hydrogen bonds from hydrogen bond donors (e.g. water, alcohols). |
|
|
|
Sulfur --> thiols, thioethers and disulfides
Physical Properties: Hydrogen bonding is much weaker than that in alcohols. Lower boiling points than similar alcohols. Generally similar to alcohols, but bonds to S are longer and weaker than those to O. The thiol functional group consists of an S atom bonded to a C atom and a H atom via s bonds. The S-H bonds is less polar than that in alcohols since S is less electronegativity than O. |
|
|
|
Amines
The amine functional group consists of an N atom bonded either to C or H atoms via s bonds. Both the C-N and the N-H bonds are polar due to the electronegativity of the N atom. The trigonal pyramidal arrangement of bonds around nitrogen is shallower in aryl amines vs alkyl amines. This is a result of resonance delocalisation of the lone pair into the aromatic p system (such delocalisation is also responsible for the decreased basicity of aryl vs alkyl amines). |
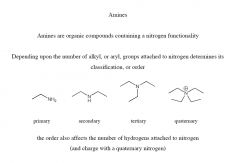
|
|
|
Carboxylic Acids Derivatives
|

|
|
|
Pharmacological Amines
|
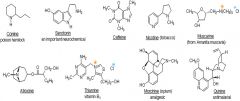
|
|
|
Esters
Cyclic esters are called lactones |
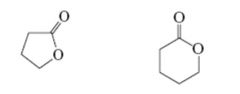
|
|
|
Anhydrides
|
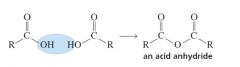
|
|
|
Amides
Cyclic amides are called lactams |
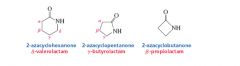
|
|
|
Carbonates, Carbamates, and Ureas
|
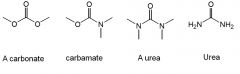
|
|
|
Functional Groups (Overview)
|
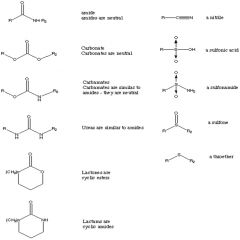
|

