![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Polar Molecule |
molecules with an overall dipole when you take into account any dipoles across the bond. |
|
|
|
Dipole |
a small charge difference across a bond resulting from a difference in electronegativities between the bonded molecules. E.g HCl |
|
|
|
Instantaneous Dipole |
Electrons are constantly in motion so at any particular time they may not be evenly distributed so one end is slightly negative and a dipole arises e.g Cl2 |
|
|
|
Nucleophile |
A molecule or negatively charged ion with a lone pair of electrons that can be donated to a positively charged atom to form a covalent bond. |
|
|
|
Substitution reaction |
A reaction in which one atom or group in a molecule is replaced by another atom or group |
|
|
|
Non Polar Bonds |
In a covalent bond where the two bonding atoms are the same, the electrons are shared equally so the bond is non polar. Eg. H-:-H |
|
|
|
Polar Bonds |
In a covalent bond where the bonding atoms do not share the electrons equally due to differences in electronegativity, atom size or nuclear charge. More electronegative atoms attract the shared electrons more becoming slightly negatively charged and the other slightly positive. |
|
|
|
Polar molecules |
A molecule with an overall dipole when you take into account any dipoles across the bond. |
|
|
|
Factors for a polar molecule |
1) Big difference in electronegativities 2) Non symmetrical shape |
|
|
|
Electronegativity |
A measure of how strongly a covalently bonded atom attracts the shared pair of electrons to itself. |
|
|
|
Dipole |
A molecule with a positive end and a negative end because of its polar bonds. |
|
|
|
Dipole-Dipole Force |
An attractive force between dipoles in neighbouring molecules. |
|
|
|
Permanent Dipole |
A small charge difference across a bond resulting from a difference in electronegativities of the bonded atoms. |
|
|
|
Intermolecular Force |
Eg. VDWF/ Permanent dipole-dipole force An attractive force between neighbouring molecules. |
|
|
|
Permanent Dipole - Permanent Dipole Force |
An attractive force between permanent dipoles in neighbouring molecules. |
|
|
|
Instantaneous Dipole - Induced Dipole Force |
-Weakest Type of intermol. Bond -The more electrons the atom has the greater the dipole effect -Same in larger atoms as they have more electrons |
|
|
|
Types Of Dipole: Instantaneous Dipole |
Oscillations of electrons within the shell mean that at any moment the distribution of charge may be uneven. One part of the molecule will be slightly negative and the other slightly positive |
|
|
|
Types Of Dipole: Induced Dipole |
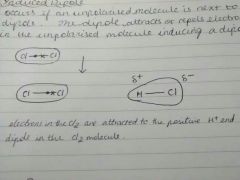
Occurs if an unpolarised molecule is near a dipole. The dipole attracts or repels electrons in the unpolarised molecule, inducing a dipole. |
|
|
|
MP & BP of Halogens |
The melting and boiling points of halogens increases down the group because at the top there are fewer electrons and the atoms is much smaller so there are weak VDWF which are easy to overcome. |
|
|
|
Mp & Bp of Alkanes |
In Alkanes the longer the chain, the stronger the intermol. forces and the higher the boiling point. In straight chain Alkanes there are more contacts between molecules and so more chances to form intermol. bonds. Thus straight chain Alkanes have higher boiling points. |
|
|
|
Ammonia as a nucleophile |

The haloalkane is heated in a sealed tube with concentrated ammonia solution. The product is an amine with an NH2 group. |
|
|
|
Factors affecting the rate of hydrolysis |
Polarity of the C-Halogen bond Bond enthalpy of the C-Halogen bond (bond enthalpy is more important) |
2 |
|
|
Common nucleophiles |

|
|

