![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Oxygen transport in mammals (OCR) |
Oxygen is transported with erythrocytes which contain haemoglobin. Binds (reversibly) to become oxyhaemoglobin. |
|
|
|
Haemoglobin (OCR) |
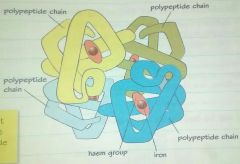
Haemoglobin has a quaternary structure: 1. 4 polypeptide chains(amino group) 2. 1 iron ion in each chain(Fe²+ attract and holds oxygen molecule) |
|
|
|
Uptake and release of oxygen (OCR) |
Ability of haemoglobin to take up and release oxygen depends on the partial pressure of oxygen(pO²) |
|
|
|
Low pressure of oxygen (OCR) |
Haemoglobin does not readily take up oxygen molecules as haem group is at the centre of haemoglobin molecule. Makes it difficult for oxygen molecule to reach the haem group and associate with it. |
|
|
|
Conformational change |
Oxygen molecule diffuses into haemoglobin molecule and associates with one of the haem group. Causes slight change in shape of haemoglobin molecule. |
Once haemoglobin molecule contains three oxygen molecules, it becomes more difficult for the fourth molecule to diffuse in and associate with the last available haem group. Difficult to achieve 100% saturation of all haemoglobin molecules, even when oxygen tension is very high. |
|
|
Haemoglobin and carbon dioxide |
85% of carbon dioxide carried in the blood is carried in the red blood cells as hydrogen carbonate ions (HCO₃-), 5% is dissolved in the blood plasma and 10% is carried by carboaminohaemoglobin (HbCO²). |
|
|
|
Chloride shift |
1. CO₂ diffuses into the RBC 2. Carbonic anhydrase enzyme (CO² combines with H²O) forms H₂CO₃ (Carbonic acid-very unstable) 3. H₂CO₃ breaks down (H+ and HCO³-) 4. HCO₃- diffuse out 5. H+ causes Hb4O₂(oxyhaemoglobin) to release O₂ 6. O₂ diffuses out RBC |
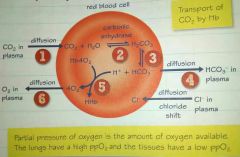
HCO₃- diffuse out the RBC into the blood plasma. This unbalances the charge of the red blood cells so Cl- ions diffuse into it to redress the balance.
|
|
|
What prevents blood from becoming too acidic? |
Hydrogen ions are taken up by haemoglobin to produce haemoglobonic acid(HHb+). |
|
|
|
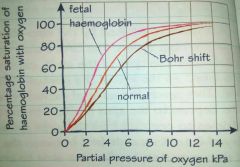
Fetal haemoglobin |
Haemoglobin of a mammalian fetus has a higher affinity for oxygen than of an adult haemoglobin. The oxyhaemoglobin dissociation curve for fetal haemoglobin it to the left of the curve for adult haemoglobin is to the right of the curve . |
|

