![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
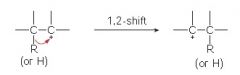
Less stable carbocations rearrange to more stable carbocations by the shift of what?
|
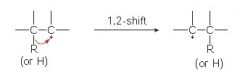
hydrogen atom or an alkyl group.
|
|
|
ROH, ROR and Epoxides contain an O atom that is
_(1)_ hybridized and __(2)__. |
(1)sp^3
(2)tetrahedral |
|
|
ROH, ROR and Epoxides have what type of polar bonds?
|
polar C-O bonds.
|
|
|
Only ___ have an O-H bond for intermolecular hydrogen bonding.
|
Alcohols
|
|
|
What two compounds do not contain a good leaving group?
|
Alcohols and Ethers
|
|
|
When can a nucleophilic substitution only occur?
|
Only after the OH (or OR) group is conerted to a better leaving group.
|
|
|
(1) Epoxides have a leaving group located where?
(2)Which makes them reactive to what? |
(1)located in a strained three-membered ring
(2)making epoxides reactive to strong nucleophiles and acids HZ that contain a nucleophilic atom Z |
|
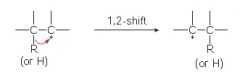
Besides rearranging, a carbocation can also react with what?
|
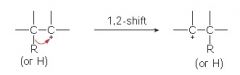
a nucleophile and a base
|

