![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
94 Cards in this Set
- Front
- Back
|
What is malonyl thioester?
|
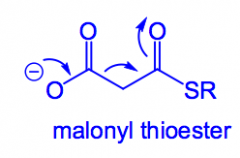
The carbanion equivalent used in the biosynthesis of fatty acids. Loss of CO2 is concomitant with nucleophilic attack, it's used to make the enolate, but technically the enolate is avoided completely.
|
|
|
How is amlonyl thioester made?
|
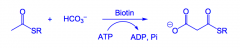
From acetyl thioester using ATP and biotin.
|
|
|
What is biotin?
|
A CO2 carrier, it transfers CO2 to another molecule (acetyl) in the synthesis of fatty acids. It's a "Carboxylation coenzyme."
|
|
|
What is ACP?
|
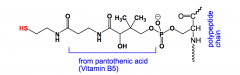
Acyl carrier protein, used in fatty acid biosynthesis to prevent the growing chain from floating away until it is deliberately attached.
Has a long arm made from pantothenic acid (& other components) and a thiol group. |
|
|
What is FAS?
|
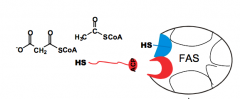
A multiple enzyme complex, Fatty Acid Synthase.
|
|
|
What is conjugate addition?
|
"Michael Addition" - the addition of a nucleophile to the beta carbon of an alpha-beta unstaturated carbonyl.
Used in the final reduction of fatty acid biosynthesis; the reduction of an alkene to an alkane by NADPH. |
|
|
What is required to elongate an existing fatty acid thioester by 2C?
|
1 acetyl CoA
1 ATP (to convert the above acetyl CoA to malonyl CoA) 2 NADPH for the respective reductions. |
|
|
What do desaturase enzymes do?
|
Introduce a cis alkene using atmospheric oxygen as the oxidizing agent. (To alter fatty acid tails.)
|
|
|
What is the omega end?
|
The methyl end of a fatty acid chain, omega 3 fatty acids have a double bond 3 carbons from the omega end (methyl); they cannot be synthesized by humans and must be consumed in our diet.
|
|
|
What do lipase enzymes do?
|
Hydrolyze triacylglycerides, these hydrolysis products can be absorbed much more easily from the digestive tract.
|
|
|
What are soaps?
|
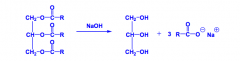
Metal salts of fatty acids, prepared by the base hydrolysis of triglycerides.
|
|
|
What is a micelle?
|
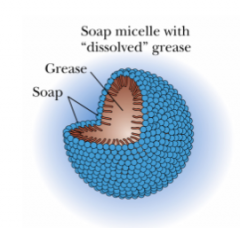
A spherical structure formed from soap molecules that have a very hydrophillic head (ionic) and a
hydrophobic (lipophilic) tail. |
|
|
What causes soap to precipitate?
|
![Very high [Na+], the equilibrium is forced to the left](https://images.cram.com/images/upload-flashcards/945781/886682_m.png)
Very high [Na+], the equilibrium is forced to the left
|
|
|
What gives synthetic detergents their advantage?
|
A head that is not a carboxylate group, it doesn't precipitate out in the presence of Ca2+, Mg2+, Fe3+, etc.
|
|
|
How do you make an anionic detergent?
|
1. acid-catalyzed, self-polymerization of propylene
2. the tail reacts with benzene by an electrophilic aromatic substitution (EAS) 3. sulfonation, another electrophilic aromatic substitution; this gives the sulfoniacid, which can be treated with a base to yield the sodium sulfonate salt. |
|
|
What is a Cationic detergent?
|
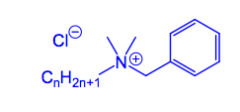
Typically a quaternary ammonium salt.
|
|
|
What are non-ionic detergents?
|
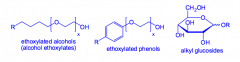
Detergents that contain a hydrophilic head without any formal charge.
|
|
|
What are silicates used for?
|
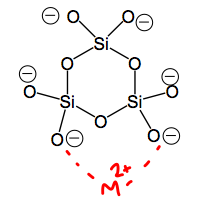
To replace phosphate based additives put into synthetic detergents.
|
|
|
What are phosphate based additives used for?
|
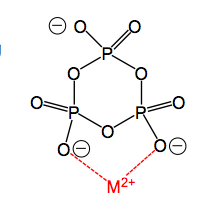
Chelating positively charged ions, prevent them from combining with clay and mud when doing laundry.
|
|
|
What are waxes?
|
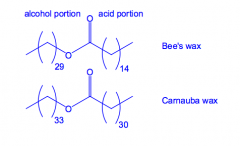
Monoesters of long chain alcohols and fatty acids.
|
|
|
What are phospholipids?
|
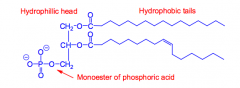
They are glycerol esterified to two fatty acids and one phosphate "phosphatidic acid." They are found in plant and animal membranes.
|
|
|
What is a prostaglandin?
|
A C20 prostanoic acid skeleton characterized by a C7 carboxylic acid chain, a cyclopentane ring, and a C8 chain.
They are synthesized from arachidonic acid only in presence of specific physiological triggers: injury or infection. Prostaglandins originate from the compound formed from the oxidation of arachidonic acid by O. |
|
|
What are Leukotrienes?
|
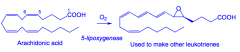
A response to allergens, syntheisized from arachidonic acid (similar to prostaglandins).
The first step in the biosynthetic pathway involves the epoxidation of arachidonic acid by the enzyme 5-lipoxygenase. |
|
|
What is a sesquiterpene?
|
A 15 carbon unit, made of 3 isoprene units connected by 2 head to tail linkages.
** two of these added tail to tail makes a triterpene. |
|
|
What are DMA-OPP and IPP-OPP?
|
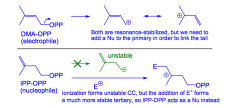
The precursors for terpene biosynthesis are C5 units: dimethylallyl pryrophosphate and isopentenyl pyrophosphate.
IPP is the nucleophile, DMA is the electrophile. |
|
|
What is Geranyl OPP
|
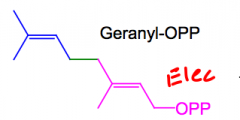
The C10 monoterpene made from DMA-OPP and IPP-OPP.
It can be used to make monoterpenes or, it can act as an electrophile and be elongated. |
|
|
What are Triterpenes & Tetraterpenes, where do they come from?
|
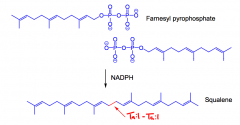
C30 and C40 units of terpenes, they are created from the reductive dimerization of 2 sesquiterpenes or 2 diterpenes (respectively).
NADPH is used in this to make the Tail to Tail connection. |
|
|
What is mevalonic acid?
|
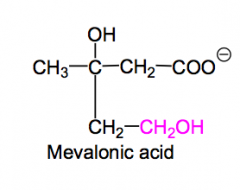
The intermediate relating the aldol condensation product and IPP-OPP.
|
|
|
What is a steroid?
What is it made from? Where are the methyl groups? |
These compounds are plant and animal lipids that consist of a characteristic tetracyclic fused-ring system: three 6-membered rings and one five-membered ring.
Rings are fused ina trans manner, H and CH3 groups are also trans to each other and in the axial posiition. Synthesized from terpenes. C10 & C13 |
|
|
What is squalene?
|
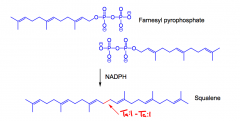
A C30 terpene, 'triterpene' made from the reaction of 2 farnesyl pyspohosphates using NADPH 'reductive dimerization.'
They are used in the biosynthesis of other triterpenes and steroids. |
|
|
What is Lanosterol?
|
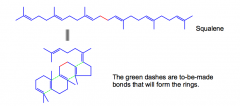
A compound used for the synthesis of many other steroids, is made directly from squalene. The C-C bonds formed aremade by the reaction of a CARBOCATION with an ALKENE
|
|
|
What is Vitamin A?
|
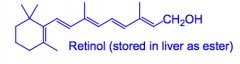
"Retinol" a fat soluble vitamin that arise from the metabolism of Beta Carotene.
It's oxidized to retinal and incorporated by as an iminie with an NH2 group in the protein Opsin. Double bond isomerization results in vision processes... |
|
|
What gives vitamin E it's antioxidant action?
|
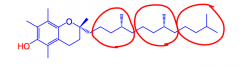
Its phenolic OH.
It easily loses H atoms (proton + electron) to give a resonance-stabilized free radical. The H atom quenches other free radicals that may damage biomolecules. The vitamin E radical is stable and hindered, so it does not cause damage. |
|
|
What is BHT?
|
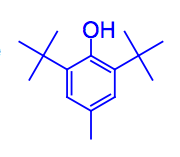
A synthetic antioxidant used as a food preservative.
|
|
|
How do H2/Pd and Br2 add to an alkene?
|
H2 is syn addition, on the side with the fewest conflicting substituents, Br2 is anti addition.
|
|
|
How is the OH group protected in DNA synthesis?
|
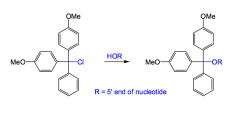
The 5’-OH group is protected as a dimethoxytrityl (DMT) ether. This ether can be prepared by the SN1 reaction of the 5’-OH with DMT chloride.
|
|
|
How are protecting groups removed in DNA synthesis?
|
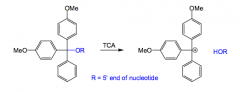
With trichloroacetic acid (TCA).
|
|
|
How is the 3' OH protected in DNA synthesis?
|
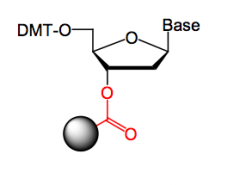
The 3’-OH group of the first unit is linked to a polymer
with a COOH group using DCC, forming an ester. |
|
|
How is the coupling reaction performed in DNA synthesis?
|
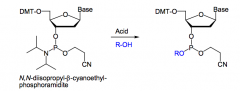
An acid-catalyzed substitution, where the amine is replaced by an alcohol (which is the 5’-OH of the attached monomer).
|
|
|
What is tetrazole used for?
|
It's an acid used in the beginning of the coupling reaction in DNA synthesis, it doesn't remove DMT or hydrolyze the ester attaching the 3' OH to the polymer. It's a weak acid, performs acid catalyze SUBSTITUTION.
THis makes the phosphoramidite which is later oxidized to a phosphate. |
|
|
How is the a phosphite oxidized to a phosphate in DNA synthesis?
|
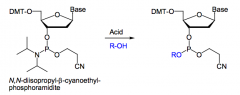
With I2 and H2O.
|
|
|
What does cyanoethyl do?
|
It protects the phsophate O- in DNA synthesis, it's not removed until the end.
|
|
|
What does aqueous ammonia do in DNA synthesis?
|
Cleaves the product from the insoluble polmer and removes the cyanoethyl groups (Beta-elimination.)
|
|
|
What are the three classes of UV light?
|
1. UV-A (320–400 nm) can cause some DNA damage.
2. UV-B (290–320 nm) is the major lethal/mutagenic part of sunlight. 3. UV-C (180–290 nm) is deadly. |
|
|
What is a T-T dimer?
|
![A UV caused form of DNA damage. Two adjacent thymines undergo a [2+2] photocycloaddition reaction that results in the formation of a cyclobutane ring. The shape of the dimer affects the structure of DNA.](https://images.cram.com/images/upload-flashcards/945781/899324_m.png)
A UV caused form of DNA damage. Two adjacent thymines undergo a [2+2] photocycloaddition reaction that results in the formation of a cyclobutane ring. The shape of the dimer affects the structure of DNA.
|
|
|
Why is benzene carcinogenic?
|
The carcinogenicity of benzene is not due to benzene itself, but rather one of its oxidation metabolites, an epoxide. Ironically, the body oxidizes benzene in an attempt to make it more soluble for excretion.
|
|
|
What are PAH's?
|
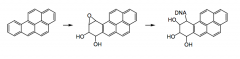
Polyaromatic hydrocarbons, a carcinogen created when cooking, (from the burning of fat). They're large, planar, hydrophobic molecules capable of intercalating with DNA.
|
|
|
What is the primary structure of DNA?
|
The covalent backbone, made of alternating units of deoxyribose and phosphate in which the 3' hydroxyl of one deoxyribose unit is joined by a phosphodiester bond to the 5' hydroxyl of another deoxyribose unit.
|
|
|
What is the secondary structure of DNA?
|
The double helix.
|
|
|
What are Glycoproteins?
|
Mainly protein with some short branched carbs, (used for receptors, recognition and cell to cell interactions.) EG antigens on bloood.
|
|
|
What are proteoglycans?
|
Long linear carbs with minor protein portions. (connective tissue, lubricatin fluid, etc.)
|
|
|
What are peptidoglycans?
|
Long linear carbs, crosslinked by short oligopeptides, found in bacterial cell walls.
|
|
|
What are Lipopolysaccharides?
|
Fatty acids linked to carbs, (outer envelope of Gram-negative bacteria.)
|
|
|
Which directions do D / L enantiomers rotate light?
|
D = dextro = right
L = levos = left. |
|
|
How do you distinguish a D monomer?
|
It has the OH group of the stereocentre furthest fromt eh carbonyl group on the right.
|
|
|
What are anomers?
|
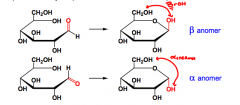
Two stereoisomers, that differ only in the configuration fo teh anomeric carbon.
Also diastereomers... |
|
|
What is mutarotaton?
|
The intercoverting of anomers in aqueous solutions.
Overtime an equilibrium mixture of both anomers will appear. |
|
|
What is a glycoside?
HOw is it formed? |
The acetal of a sugars, formed from the reaction of a HEMIACETAL with an ALCOHOL.
**SN1 REACTION in acid? (mixture of alpha/beta always formed) Glycosides are named by writing the name of the group attached to the oxygen, followed by the name of the carbohydrate, but ending in ide. These are stable in basic/neutral pH. don't revert to open chain form without acid or an enzyme. |
|
|
What happens in oxidation to aldonic acids?
|
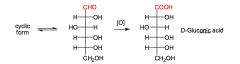
The aldehyde group of aldoses is oxidized to a carboxylic acid ! (Suffix - onic).
Both Benedicts, and Tollen's Reagents test for aldoses by making aldonic acids. |
|
|
What does Benedicts test for?
|
The presence of reducing sugars (sugars with a free aldehyde or ketone group). The sugar becomes oxidized to an acid; the cupric ion (Cu2+--->Cu1+) gets reduced; and Cu2O(s) forms, (a red solid).
Disaccharides can be oxidized if possess potential aldehydes, those connected ANOMERIC carbon to ANOMERIC carbon are acetals and cannot be oxidized by benedicts reacgent. ** False positive comes from a 2-ketose that isomerizes to an aldehyde... |
|
|
How are polysaccharides hydrolyzed?
|
H+ to H20 to their monosaccharide units.
|
|
|
What is the Tollen's reagent?
|
Ag(NH3)2 in aqueous ammonia (Ag+).
OXidation of aldehyde to acid is indicated by Ag formation (silver mirror). ** TOLLENS IS ALKALINE, because of this ketoses will slowly isomerize to aldehydes and show a positive reducing sugar test... |
|
|
How does oxidation to aldaric acids happen?
|
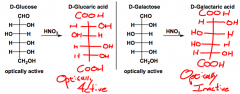
Nitric acid, is a stronger oxidizing agent (than tollens/benedict), it oxidizes both an aldehyde AND a primary alcohol to carboxylic acids...
'aric' |
|
|
What does HNO3 do?
|
Oxidizes aldehydes and primary alcohols to carboxylic acids...
|
|
|
How does oxidation to Uronic Acids occur?
What is the product used for? |
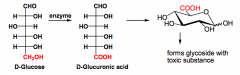
This oxidizes the PRIMARY ALCOHOL but not the aldehyde... This is performed by enzymes.
By the liver to make glycosides with toxic substances containing hydroxyl groups... |
|
|
What does bromine water do?
|
Reduces aldehydes to carboxylic acids, It's the oxidant, it becomes reduced to two Br- ions...
|
|
|
What is reduction to alditols?
What reducing agents can perform this? What is the purpose? |
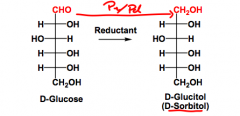
When carbonyl groups (either the aldehyde or the ketone) are reduced to alcohols.
Can be performed by H2/metal, NaBH4 or LiALH4. These make sugar alcohols that can't be absorbed well by the body; sorbitol, Xylitol. |
|
|
What happens to sugars in basic solution?
|
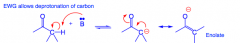
If the alpha carbon has a hydrogen, they can form enolate anions...
THIS DESTROYS STEREOCHEMISTRY. |
|
|
What is the mechanism of epimerization?
|
In basic solution, the base deprotonates the carbon that is alpha to the carbonyl, creating a carbanion that quickly converts to an alkene, giving the carbonyl oxygen a negative charge. In turn the alkene is protonated, returning the oxygen to a double bond and potentially altering the orginal stereochemistry. This is epimerization, when stereochemistry is altered at only ONE carbon.
Isomerization results in the change from ketone/aldehyde. |
|
|
What is the mechanism of isomerization?
|
Like epimerization, the alpha carbon is deprotonated, an alkene is formed with the negative charge moving up to the oxygen; but instead of the alkene being protonated the oxygen is protonated, resulting in an ene-diol.
This in turn is deprotonated, resulting in potentially the alpha carbon OH being deprotonated (instead of the carbonyl OH) and the conversion of the alkene to a carbonyl on the alpha carbon instead of the original. Two isomers, ketone/aldehyde become present over time, with the aldehyde having two possible epimers (as a result of epimerization). |
|
|
What is an aldol reaction?
|
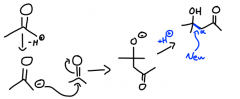
A NUCLEOPHILIC ADDITION REACTION, it forms a new carbon-carbon bond; enolates attack other aldehydes or ketones...
|
|
|
What does aldolase do?
|
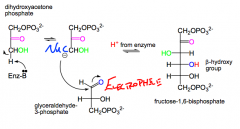
Catalyzes the reversible formation of fructose-1,6-bisphosphate in glucose biosynthesis.
|
|
|
What is a retro-aldol reaction?
|
Glycolysis: the reverse of the nucleophilic addition reaction of aldol reactions.
It destroys carbon carbon bonds... |
|
|
What is acylation?
|
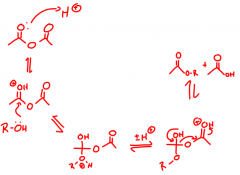
The addition of acyl groups to the alcohols to form, in this case, acetyl esters, by a nucleophilic acyl substitution reaction.
Called 'esterification' converting alcohols to esters with an acid derivative. |
|
|
What is alkylation?
What is used? What's another name? |
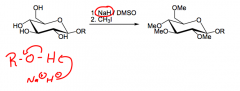
The conversion of alcohols to alkoxides in the presence of a base.
Sodium hydride for the base, (H2 bubbles away), DMSO (a polar aprotic solvent). Alkoxides are good nucleophiles, they can react SN2 with alkyl halides to form ethers... |
|
|
What is kilianai-fischer?
|
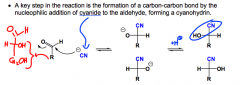
A method of synthesizing rare monosaccharides, it's a carbon-chain lengthening procedure using cyanide...
|
|
|
What is the traditional method of Kiliani-Fischer synthesis of monosaccharides?
|
Cynadie is added, forming a carboxylic acid, this spontaneously forms a lactone. The lactone is hydrolyzed by base and the diasteromeric salts are formed.
These diastereomers are separated, converted BACK to their lactones with acid (H3O+) and then reduced to aldehyde with Na/Hg amalgam... Starts as an aldehyde ends as an aldehyde, C1 in the original sugar becomes C2 in the new sugar... |
|
|
What is the modern method of monosaccharide synthesis?
|
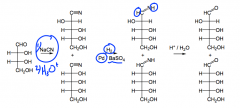
A catalyst REDUCES the nitrile to an imine, the imine is hydrolyzed, and it becomes an aldehyde....
|
|
|
What are acetal linkages?
|
The glycosidic bond of at least one anomeric carbon to a carbon in another sugar.
They are formed in acidic conditions, stable in neutral/basic. THESE ARE NOT REDUCING. |
|
|
What are maltose and cellobiose?
|
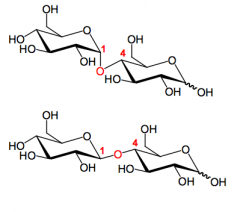
Disaccharides of glucose: alpha(1,4) and beta(1,4) respectively. They are both reducing (hemiacetals).
|
|
|
What is a lactone?
|
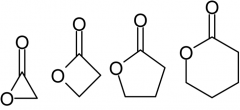
A cyclic ester, it can be oxidized like reducing sugars...
|
|
|
What is lactose?
|
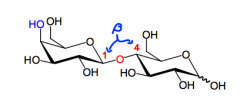
A D-galactose joined beta(1,4) to a D-glucose.
|
|
|
What is sucrose?
|
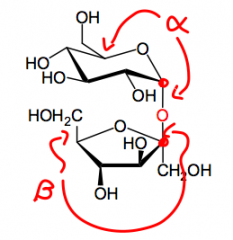
Table sugar, comprised of D-glucose and D-fructose, joined alpha(1,2) at glucose and beta(2,1) at fructose.
|
|
|
What is starch?
|
A polymer of D glucose.
The two types are amylose and amylopectin. |
|
|
What is amylose?
|
Continuous unbranched chains of D glucose joined alpha(1,4) it's a hollow helical structure.
|
|
|
What is amylopectin?
|
A branched polymer of D glucose, it has alpha(1,6) branches running along the alpha(1,4) chain.
|
|
|
What is glycogen?
|
Basically a more branched version of amylopectin, (found in animals not plants), still has alpha(1,4) and alpha(1,6) links.
|
|
|
What is cellulose?
|
A linear polymer of D-glucose linked beta(1,4). Because it's beta it can be a straight chain.
|
|
|
What is the net reaction of glycolysis?
|
1 glucose + 2NAD+ + 2PO43- +2ADP ---> 2 pyruvate + 2 NADH + 2 ATP.
C3 and C4 of glucose become C1 of pyruvate... |
|
|
What is pyruvate dehydrogenase?
|
The conversion of pyruvate to acetyl coA.
|
|
|
What are TPP and lipoic acid?
|
Coenzymes required to convert pyruvate to acetyl coA via oxidative decarboxylation...
TPP is weakly acidic, has a thiazolium ring and accepts electrons in decarboxylation. Lipoic acid oxidizes the carbon that will attach to CoA. |
|
|
What is PLP?
|
a coenzyme that functions as an amino group carrier.
It becomes PMP (pyridoxamine) after the alpha keto acid is formed. |
|
|
What is photofrin?
|
The first PDT drug, treats a form of macular degeneration. max absorption = 630 nm
The generation of O2 is highly localized to the area of irradiation, The porphyrin is essentially a catalyst, so only a small amount is needed |
|
|
What is pyridoxal phosphate?
|
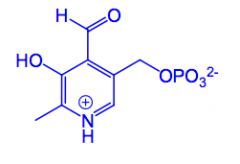
PLP a coenzyme, functions as an amino carrier, it works with an aminotransferase enzyme (ALT/AST) Aminotransferase enzymes moves the amino group
between α-keto acids and α-amino acids. PMP has an amine where the carbonyl was. |

