![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
Geometric isomers |
Cis and trans. Bonding is the same but arrangement in space is different |
|
|
Trans |

|
|
|
Cis |
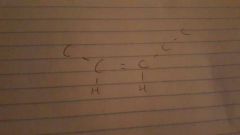
|
|
|
Alkanes |
Only single bonds between |
|
|
Alkenes |
Contain atleast one double bond |
|
|
Alkynes |
Atleast one triple bond |
|
|
Aromatics |
Cyclo, can be saturated or un |
|
|
Metane |
1 carbon |
|
|
Ethane |
2 carbon |
|
|
Propane |
3 carbon |
|
|
Butane |
C4 |
|
|
Pentane |
C5 |
|
|
Hexane |
C6 |
|
|
Heptane |
C7 |
|
|
Octane |
C8 |
|
|
Nonane |
C9 |
|
|
Decane |
C10 |
|
|
Present double bond |
Ane -> ene |
|
|
Triple bond present |
Ane -> yne |
|
|
Alcohol |
E -> ol C-C-OH |
|
|
Aldehydes |
E -> al
C-C-C=O O IS ALWAYS ON THE END |
|
|
Ketones |
E -> one O always in the middle |
|
|
Ethers |
C-O-C-C ETHYL METHYL ETHER |
|
|
Carbinicoxylic acid |
E -> oic acid Double bond O and Single bond OH at the end |
|
|
Esters |
E -> oate Carbon chains separated by O |
|
|
Amines |
E -> amines Includes N ETHYL methylamine |
|
|
Halogenalkanes |
Br, I, Cl 2,3 - diodo butane |
|
|
Substitution reaction |
Atoms replace another in a molecule |
|
|
Addition reaction |
Atom is added into an unsaturated molecule |
|
|
Condensation reaction |
2 similar molecules combine to make a large molecule and water |
|
|
Elimination reaction |
|

