![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Trigonal Planar Carbon
|
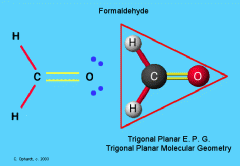
The molecule shape in which a central atom is bonded to three other atoms lying in a plane at 120 degree angles to one another
|
|
|
Molecular formula
|
A formula that shows the actual numbers of different types of atoms present in a molecule
C6H14 |
|
|
Empirical formula
|
Gives the simplest ratio of the different types of atoms in the molecule
C3H7 |
|
|
Structural formula
|
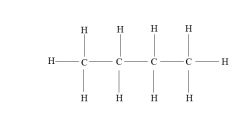
Indicates the shape of the molecules as well as the number of atoms
|
|
|
Condensed structural
|
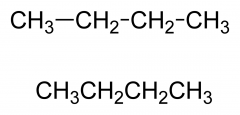
Abbreviates the formula to a single line
|
|
|
Skeletal structural
|
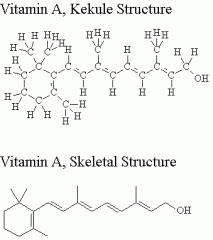
|
|
|
Configurational isomer
|
Isomers that cannot be inter converted by rotation around a single bond
The two compounds cannot be converted into one another because of the restricted rotation around the double bond- they are different compounds with different physical and chemical properties |
|
|
Equivalent hydrogen atoms
|
Gives the same H-NMR signal
Non equivalent Hydrogen's gives different H-NMR signals |
|
|
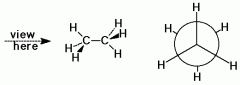
Newman Projection
|
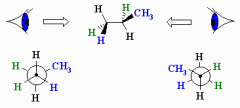
A way to view a molecule by looking along a carbon carbon bond
Short hand way of representing the staggered conformation of a molecule like Ethane |
|
|
Most stable Newman configuration
|
Staggered conformation
Minimum torsional strain, when C-H bonds of the other Methyl groups are as far away as possible from the C-H bonds of other Methyl group Minimum torsional strain |
|
|
Eclipsed Conformation
|
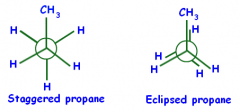
There is the maximum torsional energy when C- H bonds of one methyl group are adjacent to the C - H bonds of the other methyl group
|
|
|
Torsional strain
|
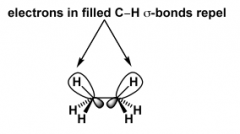
Repulsive interactions between electron clouds of the
C- H bonds on adjacent carbon atoms |
|
|
Anti -Conformation - Fully Staggered conformation
|
Lowest energy conformation , in which the 2 methyl groups are as far apart as possible with a di-hedral angle of 180 degrees
No strain |
|
|
Gauche - Staggered conformation
|
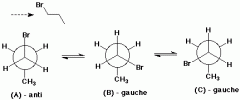
C2 & C3 bond in the anti conformation gives a staggered conformation called gauche conformation
There is no eclipsing interactions but the gauche still have 4 KJ mol higher than the anti - conformation |
|
|
Boiling points of OH
|
The boiling points of the alcohols increase as the number of carbon atoms increases
|
|
|
Primary Alcohol
|
OH group is attached to a carbon atom bonded to only one other carbon
|
|
|
Secondary Alcohol
|
The OH group is attached to a carbon atom bonded to two other carbon atoms
|
|
|
Tertiary Alcohol
|
The OH group is attached to a carbon atom which is bonded to 3 other carbon atoms
|
|
|
Nitration of Benzene ring
|
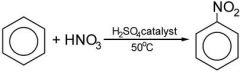
Benzene ring + HNO3 ( H2SO4 -catalyst) = Benzene & Nitro group
which can then be transferred into the amine group |
|
|
Reduction of Nitrobenzene ring
|
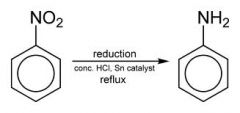
Nitro Benzene ring + H2 = Anilines
|
|
|
Carbonyl Compounds ( Ketones & Aldehydes ) + OH =
|
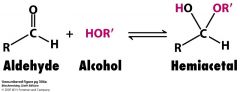
Hemiacetal
(Ketone & Aldehydes )Carboynyl + OH = Hemiacetal R-O-C-OH |
|
|
Acetal Carbon
|
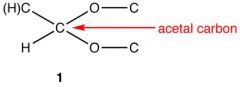
Aldehyde is reacted with an excess of alcohol - hemiacetal is sometimes not isolated - instead a acetal is formed
R-O-C-O-R |
|
|
Acid Chloride + Amine =
|

The Chloride is substituted by the NH
|
|
|
Formation of acid chlorides
|
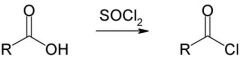
Carboxylic Acid + SOCL2 / PCl3 ----------> Acid Chloride
|
|
|
Alkene ( Double bond ) + H2
|
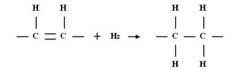
Results in an single bond
|
|
|
Chair conformations
|
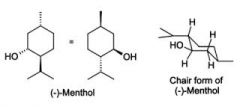
Most stable- chair conformation
|
|
|
Angle strain
|
A molecule suffers angle strain when 1 ore more angles are forced to deviate from the ideal angle, example the deviation from 109 degrees
|
|
|
Cyclohexanes - 6 membered rings
|
Are strain free due to puckering
No torsional or angle strain |
|
|
Chair conformation
|
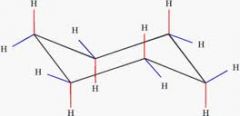
Has 2 kinds of hydrogens
Axial Equatorial |
|
|
Axial Hydrogens
|
Point up & down from the ring
|
|
|
Equatorial Hydrogens
|
Point out from the ring
|
|
|
Ring flipping
|
All Axial Hydrogens become Equatorial &
Equatorial Hydrogens become Axial |
|
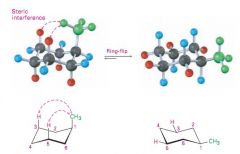
Chair conformation of methyl cyclohexane
|
The Equatorial position of Methyl group is favoured
because there are steric interactions When Methyl group is axial it experiences steric strain from interaction with the axial hydrogens on the same side of the molecule - 1 , 3 - diaxial interactions- thus the equatorial methylcyclohexane is lower in energy |
|
|
1 , 3 diaxial interactions
|
When you have a large group in the axial position , you will get 1 , 3 diaxial interactions
|
|
|
How to draw a chair conformation
1. |

1. Draw the structure of the compound using wedge/dotted line & number the carbon atoms
|
|
|
2. Draw the templates
|

2. Draw the two templates that represent the 2 chair conformations & number the carbons
|
|
|
Note :
|
Carbon atoms can either be UP or DOWN
|
|
|
Substituents represented on wedges are always positioned UP
|
The UP position on an UP carbon of the ring is axial
The UP position on a down carbon of the ring is equitorial |
|

Substituents on dotted lines are positioned down
|

The Down position on a down carbon of the ring is Axial
The down position on an UP carbon of the ring is Equitorial |
|

Axial substituents
|

Will give rise to steric strain
These conformations will have 1 , 3 - diaxial interactions that gives rise to steric strain |
|
|
Stereocentre
|
A Tetrahedral atom, most commonly a carbon with 4 different groups bonded to it
|
|
|
Carbon
|
4 Valance electrons & can form 4 single bonds
|
|
|
Nitrogen
|
5 Valence electrons & can form 3 single bonds & have 1 lone pair
|
|
|
Oxygen
|
6 Valence electrons , forms 2 single bonds & have 2 lone pairs
|
|
|
Single bonds
|
Sigma bonds
|
|
|
sp3 Carbon
|
Carbon atom that bonds to other atoms via 4 sigma bonds
|
|
|
Double bonds
|
1 Pi bond &
1 Sigma bond A consequence of the double bond is restricted rotation - which leads to cis-trans isomerism |
|
|
sp2 Carbons
|
Carbon atoms that bond to other atoms via three sigma bonds & one Pi bond
|
|
|
Tripple bonds
|
1 Sigma bond &
2 PI bonds |
|
|
sp Carbons
|
Carbons that bond to other atoms via 2 sigma bonds & 2 PI bonds
|
|
|
Bond lenghts
|
Double & tripple bonds are stronger than a single bond
The shorter the bond , the stronger the bond |
|
|
Geometries about carbon atoms
|

Linear : All the groups lie in a straight line , 180 degrees
|
|
|
Trigonal Planar
|

Three groups bonded to a carbon
|
|
|
Tetrahedral
|

Four groups attached to a carbon
|

