![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
97 Cards in this Set
- Front
- Back
|
What is allodynia? |
Pain invoked by a non-noxious stimulus |
|
|
What is somatic pain? |
MSK - Skin, muscles, bone |
|
|
Visceral pain? |
Gas pain, intestines |
|
|
What is neuropathic pain? |
Related to nerves "phantom limb" |
|
|
What is chronic pain? |
Pain that outlasts the precipitating injury or on-going pain that does not resolve Phantom limb is the extreme example |
|
|
Inflammatory pain |
A bit after a mechanical injury, when the inflammatory cytokines come in and make it swell, red etc
|
|
|
What is the pain pathway? |
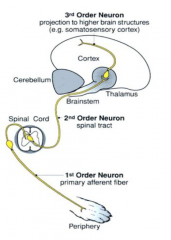
Nociceptor -> First order neuron -> Second order neuron (spinal cord) -> Third order neuron (brain)
|
|
|
Two types of nerve fibres that go to the central nervous system: |
A (fast) C (slow) |
|
|
What is glutamate? |
An excitatory AA |
|
|
What are A-delta fibres? |
For fast, sharp, surgical pain "first pain" Associated with thermal and mechanical stimuli |
|
|
What NT do A-delta fibres use? |
glutamate |
|
|
What are C fibres? |
Slow, dull, throbbing, burning pain "second pain" Unmyelinated Associated with polymodal nociceptors |
|
|
What NT do C fibres use? |
Substance P |
|
|
Where does modulation of pain transmission occur? |
In the dorsal horn of the spinal cord - receives input from ascending and descending pathways - involves alpha2, opioid and GABA receptors (among others) |
|
|
How do prostaglandins affect perception of pain? |
They sensitize the nociceptor |
|
|
Hyperalgesia is usually a _________ increase in pain |
peripheral |
|
|
Allodynia is usually due to a __________ sensitization |
central |
|
|
Why do we use analgesia during surgery and not just after you're awake? |
Give it before the event and before you perceive the pain, because once you feel it you will be more sensitive to it |
|
|
5 sites of analgesia: |
1) Peripheral nociceptors 2) Prevent transmission to spinal cord 3) Prevent transmission through spinothalamic cords 4) enhance descending inhibitory pathways 5) prevent transmission to sensory cortex |
|
|
What is the most effective way to prevent and treat pain? |
Affect all the different levels! = "balanced" or "multimodal" approach |
|
|
Lower doses of several drugs means ________ or __________ with __________ |
Means same or better analgesia with fewer side effects |
|
|
What is an opioid? |
Any substance that produces morphine-like effects |
|
|
What is an opiate? |
Any morphine-like drug with a non-peptide structure |
|
|
Opioids can act as: |
Agonists, partial agonists, and antagonists |
|
|
Opioids act at 3 key receptors: |
1) Mu* = most important - found in brain, spinal cord and peripheral - get potent analgesia, affect locomotor activity and have anti-diuretic effect, also respiratory depression 2) Delta - analgesia at spinal cord level and affect motor dysfunction 3) Kappa - get analgesia at spinal level and peripherally, cause sedation and diuresis |
|
|
What do opioids do generally? |
Decrease ascending transmission and enhance descending inhibitory pathways |
|
|
Pre-synaptic effect of opioids |
Activation decreases calcium influx in response to signal and so decreases release of excitatory NTs |
|
|
Post-synaptic effects of Opioids
|
Activation enhances potassium release, leading to hyperpolarization of membrane |
|
|
7 locations of opioid receptors |
Brainstem Medial thalamus Spinal cord Hypothalamus Limbic system Periphery Immune system |
|
|
Pure agonists have: |
high affinity for mu receptors and lower for kapa and delta |
|
|
Partial agonists can also be called |
mixed agonist-antagonists |
|
|
What do partial agonists do? |
Combine a degree of agonist and antagonist activity at different receptors, or partial efficacy Mu receptor agonist with less activity = partial agonist agonist-antagonist = kappa agonist and mu antagonist |
|
|
Antagonists are used to: |
inhibit opioid action mu antagonists |
|
|
Potency is related to: |
drug dose - how much you need to give to trigger receptor |
|
|
Efficacy is related to: |
size of the response |
|
|
Agonist drugs we use |
morphine hydromorphone meperidine fentanyl tramadol (other effects) |
|
|
Partial agonists we use |
Buprenorphine |
|
|
Agonist-Antagonists we use |
Butorphanol |
|
|
Antagonists we use |
Naloxone Naltrexone |
|
|
Pharmacologic actions in analgesia |
Supraspinal and spinal effects - if transect spinal cord, effects of morphine on preventing nociceptive spinal reflexes is less Effective in most acute and chronic pain but less so in inflammatory pain (that's why we use NSAIDs) |
|
|
Opioid effects on CNS |
1) Euphoria and sedation - mu receptor 2) Dysphoria - Kappa receptor 3) Increased locomotor activity in horses/cats (if they don't actually have pain) - mu 4) Respiratory depression - mu - worse when used in combo with anesthetics - most common cause of death in poisoning 5) Bradycardia - mu receptor - increased vagal tone 6) Suppression of cough 7) Vomiting - chemoreceptor trigger zone, varies with opiate - Morphine >>> oxymorphone 8) Pupillary constriction - centrally mediated 9) GI tract - increases tone and reduces motility - can cause constipation - Loperamide - an opiate that does not enter the brain so used as anti-diarrheal 10) Histamine release - bronchoconstriction and hypotension |
|
|
Beneficial clinical effects of opioids |
Analgesia*** Cough suppression ** Euphoria? Sedation Treatment of diarrhea (loperamide) |
|
|
Adverse side effects of opioids |
Constipation Vomiting - when you first give it Diuresis, but urine retention Respiratory depression Bradycardia Sedation Excitation - cat - horse Increased CSF pressure - cerebral vasodilation and increased carbon dioxide |
|
|
4 general uses for opioids |
Pre-anesthetic Intra-operative Post-operative Epidurals, intra-articular |
|
|
Why do we generally not used opioids orally? |
High first-pass effect! |
|
|
What routes are well-absorbed |
SC or IM |
|
|
True or false: opioids penetrate the CNS |
Generally true, but loperamide does not |
|
|
How can you restrict distribution of opioids? |
Intrathecal or epidrual |
|
|
Half life: |
30-12 minutes on average |
|
|
Duration of action |
Dose and pain dependent - usually 2-4 hours |
|
|
What is morphine? |
A mu receptor agonist - also has effects on K receptors in spinal cord |
|
|
How does morphine work? |
Raises pain threshold and alters brain's perception of pain Suppresses coughs Sedation Analgesic effect is dose depencent and we can increase effect by increase dose |
|
|
Onset of morphine |
15-30 min |
|
|
Duration of action |
Dose and pain dependent! Typically 2-4 hours Dose them at 3 hours because you don't want them to feel pain as it makes them more sensitive to it! |
|
|
What routes can you give morphine? |
IV, epidural, intrathecal IM SC continuous infusion Avoid oral |
|
|
How is morphine cleared? |
Metabolism (primarily glucoronidation) - metabolite is active |
|
|
Adverse effects of morphine |
HIstamine release - anaphylactoid reaction Vomiting Constipation Miosis (not cats) Respiratory depression - intraop (decrease response to CO2) Excitement |
|
|
What is hydromorphone? |
Primarily a mu agonist ~ 8x more potent than morphine - more commonly used as it's cheaper and easier to get |
|
|
What is hydromorphone used for? |
Sedative, analgesic, preanesthetic |
|
|
Onset of action of hydromorphone |
5-10minutes |
|
|
Duration of action of hydromorphone |
2-4 hours |
|
|
What is fentanyl |
Mu agonist - 100x more potent than morphine |
|
|
How do we usually give fentanyl? |
IV - for induction, intraoperative Skin patches available |
|
|
Onset of fentanyl |
1-2 minutes |
|
|
Duration of fentanyl |
20-30 minutes after IV |
|
|
Adverse effects of fentanyl |
Panting, bradycardia, respiratory depression after IV - can be expensive |
|
|
What Meperidine (Demerol) used for? |
Older drug, not much used anymore - okay as pre-anesthetic - too short for analgesia |
|
|
What receptor is tramadol selective for? |
mu opioid agonist actions |
|
|
What else does tramadol affect? |
Reuptake at noradrenergic and serotonergic systems |
|
|
What route should you use tramadol? |
Oral, but avoid extended release products |
|
|
What is codeine? What do we use it for? |
Moderate agonist - used as weak analgesic or an anti-tussive - most effects result of conversion to morphine - not really used in vet med |
|
|
What is hydrocodone? What do we use it for? |
Moderate agonist - primarily used as an anti-tussive in dogs - often in combo with decongestants |
|
|
What is buprenorphine? |
Partial mu agonist; mixed agonist/antagonist - 30x more potent than morphine but as effective - hard to reverse - high affinity for mu receptor so gives long duration of action |
|
|
Onset of buprenorphine |
30-60 minutes |
|
|
Duration of action of buprenorphine |
6-12 hours |
|
|
Route of administration for buprenorphine |
IM injection for cats has been approved Also good absorption across mucous membranes in cats (not dogs) - alkaline pH maintains unionized form so better absorbed (100%), but if swallowed bioavailability is about 50% |
|
|
What other species is buprenorphine also used in? Why? |
Lab animals and exotics - due to long duration of action |
|
|
What is butorphanol? |
Mixed agonist-antagonist - 4-7x more potent than morphine (but less effective) Antagonist at mu - little respiratory depression Kappa and sigma - get a ceiling effect (can't get any better the more you give) |
|
|
What happens if you give an animal on a mu agonist butorphanol? |
Reverses the analgesic effect, analgesic effect can be reduced |
|
|
What is butorphanol licensed for? |
Anti-tussive in dogs (oral) Analgesic in horses (IV) |
|
|
Onset of butorphanol |
5-10 minutes |
|
|
Duration of butorphanol |
1-2 hours |
|
|
Routes of administration for butorphanol |
IV, IM, SC but labelled for IV |
|
|
What is Naloxone? |
Reversal agent (antagonist) Competitive antagonist a mu, kapp and delta |
|
|
Duration of action of naloxone |
30-60 minutes |
|
|
Routes of administration of naloxone |
IV, IM or SQ |
|
|
Alpha2-adrenergic agonists for use in pain |
Decrease transmission of central pain response - activation of presynaptic alpha2 receptors decreases neurotransmission Main role as sedatives and analgesics - produce sedation, muscle relaxation, and analgesia - good visceral analgesia - decrease central and peripheral release of norepi - decrease sympathetic outflow and circulating catecholamines |
|
|
Drugs commonly used: |
Xylazine (Rompun)(all species) Medetomidine (Domitor) (cats and dogs, exotics) Detomidine (Dormosedan)(horses) |
|
|
Adverse effects of alpha 2 agonists |
Cardio effects - hypertension followed by hypotension - bradycardia - enhance epinephrine induced arrythmias Emesis in cats, sometimes works in dogs - Xyalzine is emetic of choice in cats Decreased intestinal motility Depresses thermoregulatory mechanisms Animals will still respond to sharp auditory stimuli |
|
|
Clinical use for alpha 2 agonists |
Restraint/analgesia for short procedures Pre-anesthetic Analgesic/sedative for colic etc Emetic in cats Only for healthy animals! Avoid in late pregnancy - cause early parturition |
|
|
Reversal of alpha 2 agonists |
Used specific alpha 2 antagonists |
|
|
Alpha 2 antagonists: |
Yohimbine - for xylazine - dogs and deer - alpha 2 selective Atipamezole - for medetomidine in dogs - alpha 2 Tolazoline - for xylazine in horses - do not use in food animals - non selective, short acting |
|
|
Anti-inflammatory effects |
Inhibition of COX |
|
|
Peripheral actions of NSAIDs |
Inflammation leads to production of prostaglandins, which sensitizes afferent nociceptors Peripheral effects of NSAIds dependent on anti-inflammatory properties However some NSAIDs have additional analgesic activities through interactions with central targets or additional mechanism |
|
|
Central action of PGs |
Release of central PGs can be inhibited by NSAIDs - they facilitate pain processing but do not act as transmitter There is evidence that effects can be reversed by Naloxone = opioid synergy |
|
|
Clinical use of NSAIDs |
Efficacy greater in inflammatory pain Efficacy greater if given prior to onset of inflammatory pain Onset and duration of analgesic actions are not necessarily the same as anti-inflammatory actions Effective dosage regimens may be different Rational to use in combo with opioids |
|
|
What is acetaminophen? |
Not really an NSAID - exact mechanism of action remains unknown Has analgesic properties Can be used in dogs (limited to dogs that don't tolerate classical NSAIDs) Effectively contraindicated in cats (glucoronidation!) |

