![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
70 Cards in this Set
- Front
- Back
|
Amino Acids What important molecules do amino acids form? |
Peptides and Proteins |
|
|
Amino Acids What are the important functions of proteins? |
Acts as... - Antibodies - Enzymes - Hormones - Structural proteins (ex. collagen, keratin) |
|
|
Amino Acids What is the general formula for an amino acid? |
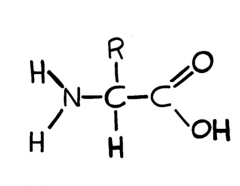
|
|
|
Amino Acids What is a peptide? |
A compound made from amino acids linked by peptide bonds |
|
|
Amino Acids What is the formula for a peptide bond? |
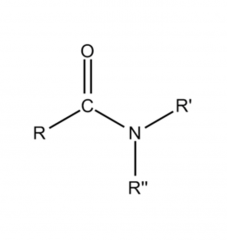
|
|
|
Amino Acids What is a Zwitterion? |
A dipolar ionic form of an amino acid formed by the donation of a hydrogen ion from the carboxyl group to the amino group. Since both charges are present, they cancel and there is no overall charge. |
|
|
Amino Acids What is the isoelectric point? |
The pH at which an amino acid exists as a Zwitterion. |
|
|
Amino Acids Draw the formation of a Zwitterion of the amino acid Glycine. |
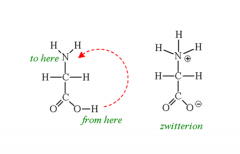
|
|
|
Amino Acids What does amphoteric mean? |
Can react with both acids and bases. |
|
|
Amino Acids Draw and explain what happens when a Zwitterion is reacted with a solution that has a more acidic pH |
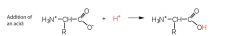
The Zwitterion behaves as a base and accepts hydrogen ions to form a positively charged ion.
|
|
|
Amino Acids Draw and explain what happens when a Zwitterion is reacted with a solution that has a more alkaline pH |
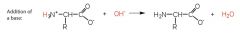
The Zwitterion behaves as an acid and donates hydrogen ions to form a negatively charged ion.
|
|
|
Amino Acids What kind of reaction occurs when forming a dipeptide? |
Condensation, you ding dong |
|
|
Amino Acids Draw the formation of a dipeptide |
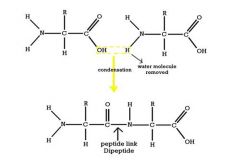
|
|
|
Amino Acids How is it possible to form a different dipeptide from the same two amino acids? |
Switch which groups you take from which Ex. Take the COOH from Amino acid 1 and bond to the OH from Amino Acid 2 or take the COOH from Amino Acid 2 and the OH from Amino Acid 1 |
|
|
Amino Acids WHAT ARE PROTEINS? (woah agressive caps lock) |
Proteins are polypeptides formed from many amino acids joined by peptide bonds in a condensation reaction |
|
|
Amino Acids How are polypeptides/proteins broken down? |
By hyrdrolysis - either acidic or alkaline. |
|
|
Amino Acids What are the conditions for the acid hydrolysis of a polypeptide/protein? |
- Reflux - 24 Hours - 6mol dm^-3 HCl |
|
|
Amino Acids What are the conditions for the alkaline hydrolysis of a polypeptide/protein? |
- 100°C - NaOH (aq) |
|
|
Amino Acids Draw the reaction for the acidic hydrolysis of a polypeptide |

|
|
|
Amino Acids Draw the reaction for the alkaline hydrolysis of a dipeptide |

|
|
|
Optical Isomerism What are stereoisomers? |
Species with the same structural formula but different arrangement of atoms in space. |
|
|
Optical Isomerism When does E/Z isomerism occur? |
When each of the carbons on the double bond is attached to two different groups |
|
|
Optical Isomerism What is an optical isomer? |
Stereoisomers that are non-superimposable mirror images of one another |
|
|
Optical Isomerism What is a chiral carbon? |
A carbon with four different atoms/groups of atoms attached. |
|
|
Optical Isomerism What is the difference between optical isomers? |
They are chemically identical, but rotate polarised light in opposite directions - anti-clockwise or clockwise. |
|
|
Optical Isomerism What is a racemic mixture? |
A mixture containing equal amounts of each optical isomer - it does not rotate polarised light because they cancel eachother out. |
|
|
Optical Isomerism Explain how optical isomers usually exist in plants and animals. |
In plants and animals, only one of the optical isomers is synthesised naturally, and only one will interact with an enzyme due to the stereospecific nature of enzymes. |
|
|
Condensation Polymerisation What is condensation polymerisation? |
The joining of two monomer units with the elimination of a small molecule - Ex. H2O or HCl |
|
|
Condensation Polymerisation What does condensation polymerisation require? |
Monomers with two different functional groups |
|
|
Condensation Polymerisation What are the two common condensation polymers you need to know? |
Polyesters and Polyamides |
|
|
Condensation Polymerisation How are esters formed? |
Carboxylic Acid + Alcohol -> Ester + Water |
|
|
Condensation Polymerisation Draw the general reaction of polysters made from two different types of monomer units - and give an example. |
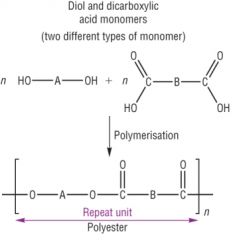
Example = Terylene |
|
|
Condensation Polymerisation Draw the general reaction of polysters made from one monomer unit - and give an example |
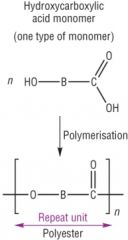
Example = Poly(lactic) acid |
|
|
Condensation Polymerisation Name 4 uses of polyesters |
1) Carpets 2) Sports clothing 3) Shirts 4) Often blended with cotton for clothing and bedding |
|
|
Condensation Polymerisation What are the properties of Polyester? |
- Strong - Resistant to stretching - Resistant to chemical attack - Burns Easily |
|
|
Condensation Polymerisation What are the properties and uses of poly(lactic) acid? |
- Biodegradable - Used for food and drink cartons |
|
|
Condensation Polymerisation What functional groups are required for the formation of a polyamide? |
- COOH - NH2 |
|
|
Condensation Polymerisation Draw the general reaction of polyamides made from one monomer unit - and give an example |
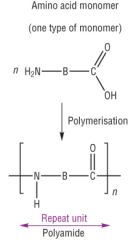
Example =Polyamide nylan-6,6 |
|
|
Condensation Polymerisation Draw the general reaction of polyamides made from two monomer units - and give an example |
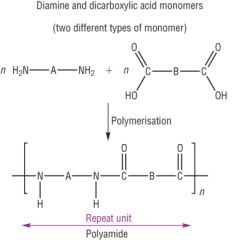
Example = Kevlar |
|
|
Condensation Polymerisation
What are the properties and functions of Kevlar? |
- Fire resistant - High strength - Used in protective clothing - crash helmets, bullet proof vests. |
|
|
Condensation Polymerisation What are the uses of polyamides? |
- Used in clothing |
|
|
Addition Polymerisation What type of compound takes part in addition polymerisation? |
Alkenes undergo addition polymerisation to produce saturated chains. |
|
|
Addition Polymerisation Describe additon polymerisation |
The polymer is amde from only one type of monomer and there is no product other than the polymer.
|
|
|
Addition Polymerisation Draw the general formula for additional polymerisation. |
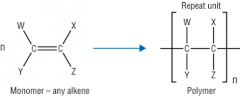
|
|
|
Addition Polymerisation vs Condensation How do you know polymerisation is Addition or Condensation? |
Addition - Monomer has a C=C double bond - The backbone of the polymer is a long chain of carbon Condensation - There are two monomers each with two functional groups - There is one monomer with two functional groups - There is an ester/amide linkage |
|
|
HYDROLYSIS Under what conditions can esters be hydrolysed under? |
Acidic or Basic |
|
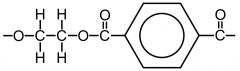
HYDROLYSIS What is formed when Polyesters are hydrolysed under Acidic conditions? Draw the hydrolysis of Terylene: |
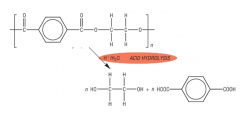
- the monomer units of the polyester are produced |
|
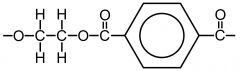
HYDROLYSIS What is formed when Polyesters are hydrolysed under Basic conditions? Draw the hydrolysis of Terylene: |
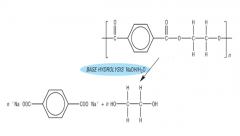
-NaOH/H2O - The sodium salt of the carboxylic acid is produced with the OH groups |
|
|
HYDROLYSIS Under what conditions can polyamides by hydrolysed under? |
Acidic or Alkali |
|
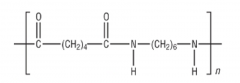
HYDROLYSIS What is formed when polyamides are hydrolysed under acidic conditions? Draw the product of acidic hydrolysis of nylon-6,6: |
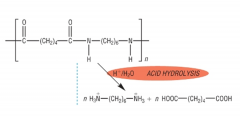
A dicarboxylic acid and the ammonium salt of the diamine H+/H20
|
|
HYDROLYSIS What is formed when polyamides are hydrolysed under basic conditions? Draw the product of basic hydrolysis of nylon-6,6: |
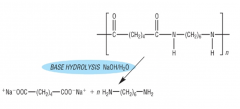
The sodium salt of the dicarboxylic acid and the diamine NaOH/H2O
|
|
|
Biodegradable Polymers What do biodegradable polymers often have? |
Chemical bonds that undergo hydrolysis |
|
|
Biodegradable Polymers Give two examples of biodegradable polymers, their origins and uses./ |
a) Poly(lactic) acid - lactic acid from corn starch, used for compost bags and food packaging. b) Poly(glycolic) acid - glycolic acid from sugar cane, used for stitches |
|
|
Biodegradable Polymers What are synthetic biodegradable polyesters used for? |
Thermofood trays and disposable plates, bowls and cups. |
|
|
Biodegradable Polymers What are photodegradable plastics? |
Synthetic polymers designed to become weak and brittle when exposed to sunlight for prolonged periods. |
|
|
Biodegradable Polymers How can photodegradable plastics be made? |
1) They are made by blending the polymer with light-sensitive additives that catalyse the breakdown of the polymer in the presence of UV radiation 2) They can be manufactured by introducing carbonyl bonds - C=O in the polymer backbone. The carbonyl bonds absorb light energy and break, fracturing the chain. |
|
|
Biodegradable Polymers What are photodegradable polymers initially converted to when exposed to light? |
Waxy compounds, before being converted to CO2 and Water in the presence of bacteria. |
|
|
Chirality in Pharmaceuitcal Synthesis Explain the case study of Thalidamide |
Thalidamide was prescribed to prevent morning sickness in pregnant women and was also used in cold and flu remedies. It is chiral - one of the stereoisomers has the desired effect but the mirror image led to deformities in developing babies. |
|
|
Chirality in Pharmaceuitcal Synthesis Explain the case study of Seldane |
Seldane was one of the first antihistamines. It relieves symptoms associated with hayfever - sneezing and nasal congestion. The 'inactive' isome caused a potentially fatal heart condition. |
|
|
Chirality in Pharmaceuitcal Synthesis What does 'pharmalogical activity' mean? |
The beneficial or adverse effects of a drug on living matter. |
|
|
Chirality in Pharmaceuitcal Synthesis How do drugs and medicines work? |
By interacting with proteins, nucleic acids and cell membranes. |
|
|
Chirality in Pharmaceuitcal Synthesis What determines the pharmalogical activity of the drug? and why? |
The 3D structure of the drug because biological molecules have complex 3D structures that can only bind to a drug molecules in one possible way. |
|
|
Chirality in Pharmaceuitcal Synthesis Why is it beneficial to have only one isomer with the correct pharmalogical activity? |
* Risks from unwanted side effects are reduced * Drug doses are reduced
|
|
|
Chirality in Pharmaceuitcal Synthesis What are the disadvantages of the seperation of racemic mixtures to get one isomer? |
X Seperation techniques are difficult because the isomers have similar properties (similar melting point, boiling point and solubility) X It is costly |
|
|
Chirality in Pharmaceuitcal Synthesis What can you use to seperate optical isomers? |
Enzymes, electrophoresis and chromatography. |
|
|
Chirality in Pharmaceuitcal Synthesis What isomers can be produced in the lab? |
A mixture of both optical isomers. |
|
|
Chirality in Pharmaceuitcal Synthesis What isomers are produced in nautre? |
A single optical isomer is produced. |
|
|
Chirality in Pharmaceuitcal Synthesis What three ways can chiral compounds be synthesised? |
1) USING ENZYMES AS BIOLOGICAL CATALYSTS Nature is good at making single optical isomers. If a synthetic route involving enzymes is designed, then a single isomer can be produced. 2) CHIRAL POOL SYNTHESIS Uses the pool of naturally occuring chiral molecules within the synthetic route. the chirality of the original molecule can lead to the formation of a product that is a single, pure optical isomer. (Common starting materials for chiral pool synthesis includes natural alpha-amino acids and sugars.) 3) USE OF TRANSITION ELEMENT COMPLEXES to produce chiral catalysts which can then transfer their chirality to synthesis a single isomer product. |
|
|
Chirality in Pharmaceuitcal Synthesis What is Ibuprofen and how does it work? |
An anti-inflammatory drug that targets bone and muscle pain. It blocks messages to the brain and reduces swelling or inflammation. It relieves heache, back pain and period pain, cold and flu symptoms and arthritus. |
|
|
Chirality in Pharmaceuitcal Synthesis Describe the chirality of Ibuprofen. |
It has one chiral carbon so has two optical isomers. One relieves pain more effectively than the other, but in the body the less active isomer is converted to the other forms. This means the full dose is active. This minimises any possible side effects. |

