![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
What is a loss of electrons called? |
Oxidation |
|
|
What is a gain of electrons called? |
Reduction. |
|
|
Reduction and oxidation happen .......... - hence the term .......... reactions. |
1. simultaneously 2. redox |
|
|
What does an oxidising agent do? |
It accepts electrons and gets reduced. |
|
|
What does a reducing agent do? |
It donates electrons and gets oxidised. |
|
|
To work out whether something has been oxidised or reduced, you need to... |
...assign each element with an oxidation number before and after the reaction. |
|

|

|
|

|

|
|
|
Metals in a redox reaction usually... |
...donate electrons to form positive ions - therefore having positive oxidation numbers. |
|
|
Non-metals in a redox reaction usually... |
...accept electrons to form negative ions - therefore having negative oxidation numbers. |
|
|
What happens to the metal and hydrogen atoms, in terms of redox, when a metal reacts with an acid. |
|
|

Show the oxidising and reducing agents in the following reaction. |
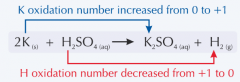
|

