![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
154 Cards in this Set
- Front
- Back
|
core of the earth
|
dense inner core (solid)of iron and nickel outer part is liquid crust (made up of tectonic plates) which float on mantel |
|
|
what does motion within this liquid core produce?
|
Earth's magnetic field which extends into space creating a protective envelope called the magnetosphere |
|
|
what is the outermost part of Earth surrounded by?
|
air- the atmospher living things- the biosphere water- the hydrosphere |
|
|
what does the atmosphere do?
|
protect from excessive solar radiation
|
|
|
what is responsible for Earth being the right temperature for water to be liquid on earth?
|
density of atmosphere distance from sun |
|
|
what provides the mechanisms for distributing energy round earth?
|
atmosphere and hydrosphere together
|
|
|
how much of earth's surface is covered by sea?
|
70%
|
|
|
what do oceans contain?
|
80% of living organisms on earth
|
|
|
what do oceans transport a lot of?
|
energy and materials
|
|
|
what started the science of oceanography?
|
HMS Challenger
|
|
|
what did they do?
|
investigated depths of oceans currents (direction and speed) took samples |
|
|
what was most notable finding?
|
how oceans control global climate- through temperatures and salinity measurements
|
|
|
why may accurate data be of little use?
|
if system studying changed significantly by end of study
|
|
|
why are satellites good?
|
great spead at carrying out extensive surveys
|
|
|
negatives?
|
less accurate
|
|
|
what have studies shown about ocean depths?
|
vary considerably
|
|
|
most important role of oceans
|
control of climate (with atmosphere) regulatory role |
|
|
secondary role?
|
storehouse of food and chemicals
|
|
|
why is it difficult to study oceans?
|
so big
|
|
|
when can erros become significant?
|
when values scaled to ocean-size eg Fritz Haber
|
|
|
what did Haber do ammonia...?
|
Ammonia process (explosives for WW1)
|
|
|
other (failure)?
|
thought could pay off Germany's war debt by extracting gold (gold compunds)from sea but calculations way smaller |
|
|
what has man being doing for thousands of yeRS?
|
using sea as valuable source of materials
|
|
|
how is bromine extraceted from sea?
|
displacement- redox reaction chlorine gas bubbled through sea water Cl2(g) + 2Br-(aq) 2Cl-(aq) + Br2 (aq) |
|
|
what is seaweed used for?
|
fertilizer source of iodine |
|
|
what percentage of substances dissolved in sea water are ionic?
|
99% |
|
|
why strictly speaking does the sea contain a mixture of ions and not "salts"
|
ions free in solution
|
|
|
what is the composition of sea water like across the globe?
|
similar
|
|
|
where are the areas of low level salinity?
|
close to freshwater: esturaies icebergs high rainfall areas |
|
|
high salinity?
|
equator rate of evaporation high hot, windy and dry climate |
|
|
2 most abundant ions in sea water?
|
sodium chloride |
|
|
where also found and vital for?
|
found in human body vital for human health (excreted via urine, replaces must) |
|
|
what are some exaples of modern sources of salt
|
underground deposits (salt mines) controlled boiling of sea water |
|
|
how are other salts which would spoil flavour removed?
|
by differences in solubility
|
|
|
how does CaSO4 precipitate out?
|
precipitates out early as water starts to evaporate and can be "skimmed off" |
|
|
taste of magnesium salts?
|
bitter
|
|
|
solubility of magnesium salts in comparison with sodium chloride?
|
more soluble so NaCl precipitates out first and can be skimmed off before magnesium salts appear |
|
|
so summary of solubility?
|
CaSO4 NaCl magnesium salts |
|
|
basic cyle, starting from water falling as rain?
|
1) water falls as rain 2) rivers carry ions in solution tp sea 3) sea is storehouse of dissolved ions 4) water evaporates from sea |
|
|
how are compounds containin mainly calcium, magnesium, carbonate and silicate ions transported to sea? |
leached from soil (rain falls, dissolves, tranports to sea)
|
|
|
where do lava and gases well up from?
|
mantle at the mid-ocean ridge
|
|
|
what are volcanic gases rich in?
|
chlorine bromine sulphur compounds |
|
|
how are else are ions produced?
|
sea water enters cracks in solidifying lava water is superheated and dissolves many minerals |
|
|
where do sodium ions come from too...
|
volcanoes (explained as above)
|
|
|
sources of dissolved ions in sea water pic?
|
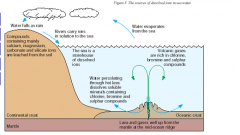
|
|
|
what is responsible for distinctive sea side smell?
|
dimethyl sulphide (CH3)2S |
|
|
how is DMS produced?
|
dimethylsulfoniopropionate DMSP is a metabolite found in marine plankton and seaweeds, and algae
|
|
|
what happens when these organisms die?
|
bacteria convert DMSP into DMS DMSP + (CH3)2 |
|
|
what then happens to this DMS?
|
released into atmosphere
|
|
|
How do DMS molecules contribute to climate regulatoin
|
main sources of cloud condensation nuclei (small molecules in atm, which encourage water molecules to bind together to form clouds) which then reflect solar radiation back into space |
|
|
what is DMS thought to contribute towards?
|
acidic pollution including acid rain and acidification of oceans
|
|
|
why?
|
as can be oxidised in atmosphere to produce acidic S compounds
|
|
|
what can it be a significant contributor to?
|
acidic rain
|
|
|
what did this compound prove to be in the global sulphur cycle?
|
missing link
|
|
|
what do phytoplankton contain and how do they get their energy?
|
chlorphyll photosynthesis |
|
|
why are phytoplankton important?
|
food web responsible foroxygen present in earths atmosphere |
|
|
what does chlorophyll absorb?
|
blue and red light (why plants appear green)
|
|
|
what does the colour of the sea indicate?
|
number of phytoplankton present green=lots blue=few |
|
|
how do scientists use this info?
|
to create world wide maps of chlorophyll from satellite images of ocean colour
|
|
|
what does this map info provide?
|
knowledge about how well phytoplankton growing in various areas
|
|
|
problems caused by rivers?
|
rivers wash mud and dead vegetation into sea close to land care taken to avoid false conclusions |
|
|
how may global warming be reduced?
|
finding out which bacteria contain one or both of the genes which produce DMS or other Sulfur containing compounds and thus more understanding about sulphur cycle and so reduce global warming |
|
|
what do some scientists predict about the concentrations of atmospheric carbon dioxide gas levels?
|
double in 100 years
|
|
|
what are the three main ways of redcing build up of CO2? 1? |
use alternative methods to produce energy instead of using fossil fuels (DF)
|
|
|
2?
|
use fossil fuels more efficiently
|
|
|
3?
|
capture and store carbon dioxide
|
|
|
what are the 4 different methods of the revolutionary CCS? 1? |
turning CO2 into useful products (as yet unknown)
|
|
|
2?
|
growing more trees and increasing organic content of soil
|
|
|
3?
|
storing gas in deep natural trenches on sea floor where pressure will cause it to liquify |
|
|
4?
|
injecting CO2 onto sea floor at depth of 3500m where form liquid CO2 lake should remain undisturbed |
|
|
pro vs con?
|
may disturb natural environment but less damaging than allowing it to be absorbed into surface waters naturally |
|
|
what happens to the solutbility of gases as temperature increase?
|
solubility decreases (why cans epolde if left in sun, less explosion if fridge...)
|
|
|
what does high pressure cause more of?
|
gas to dissolve (pop)
|
|
|
what conditions are thus needed for earth to maintain a stable environement?
|
low temperature high pressure (more gas dissolve) |
|
|
how is uptake of CO2 by oceans increased?
|
by ocean life- although is already a speedy process!)
|
|
|
what is the solubility of CO2 like compared with oxygen and nitrogen?
|
more soluble
|
|
|
why?
|
contain polar C=O bonds, which can hydrogen bond with water some carbon dioxide molecule (HCO3-) react chemically with water so removed from the equilibrium so more CO2 dissolves to maintain eq position |
|
|
equation 1?
|
CO2 (g) eqp CO2(aq) (1) ΔH is negative (exothermic, so colf temps favoured for CO2 aq)
|
|
|
2?
|
CO2 (aq) + H2O(l) <> HCO3-(aq) + H+(aq) (first to H2CO3, then as this is a weadk acid, slight dissociates into HCO3- + H+) |
|
|
3?
|
HCO3-(aq) <> CO32-(aq) + H+(aq) dissociate to form carbonate ions |
|
|
what does it mean, because equations 1-3 are linked?
|
if add or removing something, effects others too
|
|
|
what helps move the position of equilibrium shift to the right? (CO2 (g)<>CO2 (aq) ?
|
reactions 2 and 3 cause more CO2 to dissolve from the air (in reaction1) to replace that lost with the reactions in water movement of water in oceans means thatd dissolved CO2 sinks, stored for 100s of years |
|
|
what do phytoplankton also do?
|
increase rate at which CO2 dissolves in water (near surface) during photosynthesis |
|
|
how is CO2 released by phytoplankton?
|
respiration at night (CO2) phytoplankton is eaten and metabolised by animals which release CO2 back into water all goes back into atm (CO2) |
|
|
what is this an example of (system- open or closed?)
|
an open system material can enter or leave a system preventing establishment of equilibrium |
|
|
why has the ocean pH stayed nearly constant for so long?
|
ocean acts as a buffer
|
|
|
what is the equilibrium equation for this buffer?
|
CO2(aq) + H2O (l) eqm HCO3- (aq) + H+ (aq)
|
|
|
what is the weak acid?
|
carbon dioxide and water
|
|
|
is this abundant?
|
yes
|
|
|
what about its salt HCO3- ?
|
not seem to be present in large excess, so therefore would mean that the ocean would not be a good buffer, but the ocean is a good buffer |
|
|
so why so much HCO3- present?
|
if H+ added to the system solid calcium carbonate held in shells and limestone can dissolve to supply extra HCO3 - |
|
|
why is this good? |
allows equilibrium to move back to left as HCO3- reacts with H+ to form water and carbon dioxide |
|
|
what does this do?
|
maintains pH so effectively HCO3- is in large excess and ocean acts as a buffer |
|
|
what is the solubility of calcium carbonate like?
|
sparingly soluble in water
|
|
|
equation for this reaction?
|
CaCO3(s) Ý Ca2+(aq) + CO32-(aq) AH = -11 kJ mol-1 |
|
|
what type of reaction is the forward reaction?
|
exothermic
|
|
|
so for the CaCO3 to dissolve, what must happen?
|
the temperature must decrease, as this will shift the eqm position to the left so CaCO3 dissolves
|
|
|
so why does CaCO3 dissolve in shallow depth?
|
little CaCO3 dissolves before water becomes saturated with products, as already much calcium ions (CA2+ and carbonate ions) and dissolving stops, and eventually may crystallise out... also equilibrium favours lhs at higher temperatures (endothermic) |
|
|
so what does this mean?
|
no shells found at sea bed in very deep ocean, as all dissolved
|
|
|
ocean surface
|
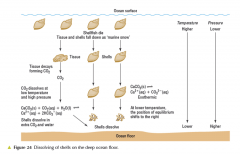
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oceans 5
|
|
|
|
what do the oceans play a vital role in?
|
circulating energy round the globe
|
|
|
how is the sun's energy spread out?
|
over a larger area nearer the poles, more intensely near equator as of Earth's tilt
|
|
|
what percentage of sun's energy reflected?
|
30%
|
|
|
absorbed by land and oceans?
|
47%
|
|
|
absorbed by atmosphere?
|
23%
|
|
|
what have temperature differences caused?
|
currents set up in ocean and atmophere
|
|
|
why?
|
spread out heating effect of sun more evenly
|
|
|
does water also play an important role in transferring heat energy round Earth?
|
yes
|
|
|
why
|
the evaporation of water is endothermic and condensation of water vapours is exothermic |
|
|
why endothermic?
|
H2O(l) H2O(g) need to overcome IMB |
|
|
why exothermic?
|
as strong new intermolecular attractions (bonds) formed (hydrogen)
|
|
|
so what does water evaporating do?
|
absorbs energy
|
|
|
condensing?
|
releases energy
|
|
|
describe how much energy would be lost from planet with no atmosphere or oceans |
incoming solar energy at any point is balanced by energy lost to outer space
|
|
|
percentage of energy lost to outer space at tropics on Earth (atmosphere and ocean)? |
80% |
|
|
what happens to other 20%? |
20% transported to colder regions by: 17% of energy recieved at tropics transported by water vapour to colder parts of earth 3% transported by warm water currents |
|
|
water cycle |
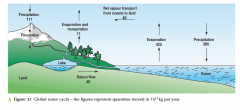
|
|
|
what is the amount/ volume of water vapour excess from oceans (to land)? |
40x 10^15kg yr-1 |
|
|
what does this water vapour excess from the oceans do? |
keeps rivers flowing lakes filled warming the land (as water vapour condenses) |
|
|
what property does water have? |
a high specific heat capacity |
|
|
what does this mean? |
large amounts of heat energy are needed to raise the temperature of 1kg of water by 1 degrees centigrade |
|
|
why? |
due to large amounts of heat energy needed to overcome strong hydrogen bonds o fwater |
|
|
specific heat capacity of propanone like? |
lower than water |
|
|
so how much rainfall would need to occur for same warming effect as water? |
4x amount as of lower specific heat capacity so less amount of energy released as condesation occurs (forming of new bonds, exothermic) |
|
|
why is water one of best liquids for transporting eergy? |
high specific heat capacity |
|
|
what causes water to be moved round the world? |
giant current circulations |
|
|
density of warm, salty (saline) water? |
low |
|
|
flow of warm, salty, water like? |
on surface of oceans to poles |
|
|
what happens when the warm water reaches the poles? |
its cooled |
|
|
what happens to density of saline water as it cools |
increases in density |
|
|
so? |
sinks underneath warm ocean currents and flows in opposite direction |
|
|
what warms up the climate of Britain? |
Gulf stream current flowing up from Carribean |
|
|
what drives the currents? |
density differences of water |
|
|
what are the charachteristics of the deeper current like? |
colder, more saline and denser water |
|
|
how fast do deep currents flow/ move? |
slowly |
|
|
how long may it take for these deep currents to reach teh surface? |
over 1000 years |
|
|
how long will the Carbon dioxide in this water be out the way for? |
over 1000 years |
|
|
why do movements of ocean currents need to be understand, to predict for what?
|
to predict climate change
|
|
|
what would happen if Greenland's ice melts?
|
resulting low salinity cold water could cut off the Gulf stream, causing North Europe to become much colder
|
|
|
what happens to frsh water (from ice melting) even if cooled below 4 degrees?
|
doesn't become denser and will not sink |
|
|
what would this do?
|
disrupt the conveyor belt
|
|
|
what happens to the solubility of CO2 as temperature increases?
|
carbon dioxide becomes less soluble in water
|
|
|
what do some climate models predict?
|
the capacity of the ocean for absorbing carbon dioxide could fall by up to 50% as ocean gets warmer, (further increasing green house effect and climate)
|
|
|
what is El Nino?
|
phenom that occurs when climatic conditions alter the currents in the Pacific to prevent cool nutrient rich water rising to the surface near Ecuador and Peru |
|
|
what does this affect?
|
fish stocks and severe weather in Australia and South US |
|
|
how may global warming be affecting EL Nino?
|
worsening it
|
|
|
what does El Nino cause in Asia?
|
monsoons
|
|
|
|
|

