![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
102 Cards in this Set
- Front
- Back
|
Jones Reagent Test
|
- corrosive
(+) Test indicates: 1 or 2 alcohols, amines, aldehydes, and phenols False (-) : 3 alcohol Solution turns from orange to blue-green |
|
|
Bromine Test
|
- Toxic
(+) test indicates pi bonds, alkenes, aldehydes and phenols Solution goes from brown to clear False (+) : Dilution False (-) : Too much reagent |
|
|
2,4-DNP Test
|
- Corrosive
(+) test indicates aldehydes and ketones Solution will contain precipitate when reacted with. False (+) - Acetone washed glass False (-) - Aromatic/hindered ketones |
|
|
FeCL3 Test
|
(+) test indicates phenol.
The solution goes from yellow to some other color. False (-) - Sterically hindered phenols |
|
|
Ignition Test
|
(+) test indicates aromatic ring (ie benzene)
Sooty/smoky flame will indicate a positive test. False (+) - Alkyl Halides False (-) - Aromatics with many oxygens |
|
|
Briefly explain how one can monitor the progress of a reaction by TLC.
|
The reactants, reactant mixture and product are placed on the TLC sheet.
After some time has passed, the dots will have moved and aligned. The reaction is complete when the reaction mixture has only one dot and is aligned with the product. |
|
|
What solvent will be used in the Diels-Alder reaction experiment, and what safety hazard does it present?
|
Toluene - it is very flammable
|
|
|
Why do cyclic dienes typically undergo Diels-Alder reactions more quickly?
|
They are permentally fixed in a cis configuration.
|
|
|
How will the product from today's experiment be isolated?
|
By filtration
|
|
|
Why is it not a good idea to clamp both the round-bottom flask and the condenser in the Diels-Alder experiment?
|
It will allow air to leak into the funnel.
|
|
|
Briefly explain what is meant by "heating at reflux"
|
Boiling a liquid and attaching a means to recollect the evaporate so that it is constantly vaporizing and boiling.
|
|
|
Why is using a cracked filter flask more dangerous than using a cracked Erlenmeyer flask?
|
The fliter flask is under extreme pressure due to the vaccuum.
|
|
|
Why is it necessary to remove the stopper from the seperatory funnel before draining it?
|
The stopper inhibits air flow and will not allow the stopcock to release the liquid.
|
|
|
Briefly explain why water was sufficient to pull the copper chloride away from the benzil, but 1M NaOH was required to seperate the p-ter-butylphenol from the benzil
|
Water is a great way to pull ionic bonds apart due to its polarity, but NaOH is more basic than water and pulled the acidic H+ from the alcohol group.
|
|
|
Why do cyclic dienes act faster than trans dienes?
|
Cyclic are always held in cis, making them more reactive.
|
|
|
What is the purpose of the watercool condenser
|
Acts as an aircoil
|
|
|
About how long did the Antrocene/maleic anhydrous heat at reflux?
|
About one hour.
|
|
|
Extraction of benzoic acid from mixed solution of benzophenone and benzoic acid
|
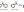
What is happening in this reaction?
|
|
|
Based on your data, which compound (napthalene, BHT or sodium benzoiate) is the most polar?
Which is the least polar? |
Sodium Benzoate is the most polar because it fully dissolved in water, a polar sovlent. Also it has a slight solutibity in Diethyl ether, which has some polarity.
Napthalene is the least polar. It was not soluble in H2O, but it was soluble in Petroleum Ether and Diethyl Ether. |
|
|
Based on the structures of the compounds, which compound (napthalene, BHT or sodium benzoate) is the most polar?
Which is the least polar? |
Sodium Bensoate is the most polar because of the depronated carboxylic acids and the Na+ ions.
napthalene is the least polar because it has no Oxygens or Nitrogens, or any type of dipole. It is strictly a hydrocarbon cyclic. |
|
|
London Dispersion
Dipole-Dipole |
List all of the intermolecular forces present in pure solutions of each compound.
|
|
|
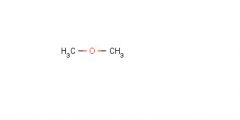
London Dispersion
|
List all of the intermolecular forces present in pure solutions of each compound.
|
|
|
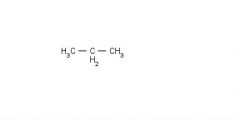
London Dispersion
Dipole - Dipole Hydrogen Bonding |
List all of the intermolecular forces present in pure solutions of each compound.
|
|
|
Which compound (dimethyl ether, propane or ethanol) would have the highest boiling point?
The lowest? |
Ethanol
Propane |
|
|
Which compound (dimethyl ether, propane or ethanol) would be the most soluble in water?
The least? |
Ethanol
Propane |
|
|
What is one good way (in terms of technique) to try to dissolve a solid in a small amount of solvent in a test tube?
|
Put a cork stopper on the test tube and shake it up
|
|
|
Predict the best solvent:
Napthalene (nonpolar) should be most soluble in |
Petroleum Ether
|
|
|
Predict the best solvent:
BHT (polar aprotic) should be most soluble in.. |
Diethyl ether
|
|
|
Predict the best solvent:
Sodium benzoate should be most soluble in... |
Water
|
|
|
If a mixture of ether and water is placed in a seperatory funnel, which layer would be on the bottom?
|
Water
|
|
|
Extraction is a seperation technique. On the basis of what physical property are seperations achieved?
(In other words, differences in what property are used to separate mixtures of compounds when doing an extraction?) |
Solubility.
|
|
|
In today's experiment, a mixture contianing the three compounds, benzyl, p-tert-butylphenol and copper (II) chloride, will be added to a mixture of ether and water.
Which layer (water) will each compound end up in? |
Cu (II) Chloride
|
|
|
In today's experiment, a mixture containing the three compounds, benzyl, p-tert-butylphenol and copper (II) chloride, will be added to a mixture of ether and water.
Which layer (ether) will each compound end up in? |
benzyl
p-tert-buytlphenol |
|
|
What is the equation for P.C?
(water partition coefficient) |
P.C = {(Amt of compound in better solvent)/(volume of better solvent)}
_________________________ {(Total amt)-(amt in better solvent)/(volume of worse solvent)} |
|
|
After the first extraction (of water and ether) the compounds remaining in the ether layer are extracted with 1 M naOH (a strong base).
One of the structures changes and this compound is 'dragged' into the new water layer. Draw the new structure and explain why this compound is suddenly soluble in water. |
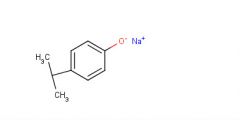
This structure had it's H+ stripped away, forming an ionic bond with Na+ and making it soluble in water because of this ionic bond.
|
|
|
What is the melting point of Octane according to ChemFinder?
|
125.5 °C
|
|
|
What is the melting point of isooctane according to ChemFinder?
|
99.0 °C
|
|
|
Why does isooctane have a lower boiling point than octane?
|
As surface area increases, van der waals forces increases, thus raising the boiling point.
|
|
|
When bubbles first start coming out of the boiling point microcapillary, why is the temperature of the oil not taken to be the boiling point of the liquid?
(In other words, why do you have to wait until the bubbles become a steady stream, and then stop when the oil is cooled? |
The bubbles began to form because of the huge increase in kinetic energy, but this does not determine exactly what the boiling point is.
The pressure is higher outside the tube than inside, and once the pressure equalizes and the temperature decreases, the boiling point can be determined. |
|
|
Would you expect the boiling point of 1-heptanol to be higher or lower than that of octane?
|
1-heptanol will have a higher boiling point because of the alcohol group that contains hydrogen bonds, as well as dipole-dipole interactions
|
|
|
What is recrystallization
|
Technique which seperates based on solubility.
As temperature increases, so does solubility. |
|
|
T/F - Slowly cooling will yield more pure product.
|
TRUE -
|
|
|
What was dimethyl maleate acting as in the alkene isomerization lab?
MP 91-99 degrees C |
Starting material
|
|
|
What was dimethyl fumerate acting as in the alkene isomerization lab?
MP 101-102 deg C |
Product
|
|
|
What was morpholine acting as in the alkene isomerization lab?
|
Nucleophile
|
|
|
What was ZnCl2 acting as in the alkene isomerization lab?
|
Lewis acid catalyst
|
|
|
What was methanol acting as in the alkene isomerization lab?
|
Solvent
|
|
|
What was diethyl ether acting as in the alkene isomerization lab?
|
Used in extractant
|
|
|
Stationary Phase is also known as what?
|
Absorbant Phase
Used Silica Gel (very polar) for chromatography. |
|
|
Mobile Phase is also known as what?
|
The solvent.
In the chromatography lab, it was Ethyl acetate . |
|
|
Rf equation
|
Distance spot traveled
---------------------- Distance solvent traveled |
|
|
Solubility
|
dissolving a substance into a liquid
|
|
|
Solvent
|
The liquid that does the dissolving
|
|
|
Solute
|
The substance being dissolved
|
|
|
Which intermolecular force is the strongest?
- Ionic interactions - Hydrogen bonds - Dipole - dipole interactions - London dispersion |
They are ranked according to their strength relative to london dispersion
|
|
|
Distillation seperates based on....
|
differences in boiling pointl.
|
|
|
Simple distillation involves...
|
An open tube that vaporizes the solution only once.
|
|
|
Fractional distillation involves
|
more surface area, which makes the solution vaporize and condense several times.
|
|
|
Normal phase
|
Silica gel -> very polar
seperates polar compounds |
|
|
Reverse phase
|
Composed of (CH3)18
Very nonpolar Located inside of the GC tube. Seperates Nonpolar compounds |
|
|
As impurities increase...
|
melting point decreases
|
|
|
What color is benzil?
MP? |
yellow
95 deg C |
|
|
What color is p-tert-butylphenol?
MP? |
White
99.3 deg C |
|
|
What is atmospheric pressure
|
The temperature at which a vapor pressure is above a liquid
|
|
|
As atm decreases....
|
BP decreases too
|
|
|
Temperature is a measure of ______ in particles.
|
Kinetic energy
|
|
|
In Experiment 4: TLC what is the stationary phase on the TLC plate?
|
The silica gel plate
|
|
|
In Experiment 4: how will we visualize spots on the TLC plate
|
Using UV light
|
|
|
What will be the result of the following errors in TLC technique?
Too much sample is applied |
Larger spots than normal will appear, will not be able to calculate Rf
|
|
|
What will be the result of the following errors in TLC technique?
Solvent of too high polarity |
The spots will be too close to the top.
|
|
|
What will be the result of the following errors in TLC technique?
Solvent in developing jar too deep. |
The samples will mix together and skew results
|
|
|
What will be the result of the following errors in TLC technique?
Forgetting to remove TLC plate when solvent reaches top of TLC plate. |
The Rf value cannot be determined
|
|
|
For Experiment 4: TLC, would your Rf values have been higher or lower if you had used a more polar solvent than ethyl acetate as the developing solvent?
|
The Rf values would have been lower if a more polar solvent was used because it would have moved the solutions closer to one another, thus lower Rf
|
|
|
For Experiment 6, Alkene Isomerization
Could a catalytic amount (less than one equivalent) of morphone be used? |
Yes it could because the end product of the reaction is dimethyl fumerate and morpholine...
This allows it to catalyze other dimethyl maleate molecules. |
|
|
How is the product from Experiment 6: Alkene Isomerization going to be seperated from the reaction mixture?
|
It's going to be put into a seperatory funnel that contains water and ether.
|
|
|
What physical property will be used to distinguish dimethyl fumerate from dimethyl maleate?
|
The melting point will increase.
|
|
|
RWhat effect is recrystallization expected to have on the melting point of your product?
|
The melting point should increase because crystallization favors creation of the sought after molecule and eliminates impurities...which lower the melting point.
|
|
|
Why does the addition of the 10% H2SO4 result in the formation of a precipitate?
|
It pulls off the Na+ ion and adds an H+. This changes the composition of the solution and forms precipitate because it's a neutral compound.
|
|
|
Recrystallizatino seperates compounds based on what physical characteristic?
|
Solubility
|
|
|
When doing a recrystallization, what is the purpose of cooling the crystallizing solution?
|
To "crash out" product
|
|
|
When dissolving the crude sample in hot solvent, why is it important to not use too much solvent?
|
The solvent may deprotonate the molecule that we are trying to isolate.
|
|
|
After dissolving the crude sample in hot solvent, why is it better to let the solution cool slowly rather than quickly?
|
This yields a purer product, quickly cooling will lead to impurities.
|
|
|
When "washing" the purified product (which has been collected in the filter) why should cold water be used instead of warm water?
|
The cold water will not dissolve the product, leaving us with a full collection of the product.
|
|
|
Which compound (n-octane or isooctante) had the shorter retention time?
|
Isooctane had the shoerter retention time beasue it was less nonpoalr than n-octane, which allowed it to move quicker through the Gas Chromotagraphy chamber.
|
|
|
What is a way to determine if isomerization was complete?
|
Detemine how close the experimental melting point is from the theorhetical melting point.
|
|
|
Why is fractiona distillation more effective than simple sitillation for seperating liquids?
|
Fractional requires that the liquids condensce multiple times, ensuring thatt the liquid is highly seperated
|
|
|
Distillation seperates compounds based on what physical property
|
Boiling point
|
|
|
What is one advantage of fractional distillation relative to simple?
|
It ensures that the liquid is more seperated than simple.
|
|
|
What is one disadvantage of fractional distillation relative to simple?
|
It takes much longer.
|
|
|
What will be the mobile phase (gas carrier) in the gas chromatography experiment?
|
SiCH2(CH3)18
|
|
|
The gas chromatograph we will use today has a nonpolar column.
Which compound will flow through the column at a faster rate? |
Isooctane
|
|
|
What information is gained from measuring the relative areas of peaks in a gas chromatogram?
|
The % composition
|
|
|
What does the number of peaks in a gas chromatogram tell you about the composition of the sample?
|
The number of peaks equals the number of compounds in the sample.
|
|
|
Explain why impurities lower the melting point.
|
impurities disrupt the bonds of the substance we're testing.
|
|
|
During this experiment you will be monitoring the temperature of the silicone oil bath.
When will the temperature of this bath equal the boiling point of your unknown? |
When the bubbles stop coming out of the microtube.
|
|
|
If a storm moves in and the atmospheric pressure drops, will the observed boiling points of the unknowns increse, decrease or stay the same?
|
The boiling points will decrease.
|
|
|
Dimethyl maleate
Starting material |
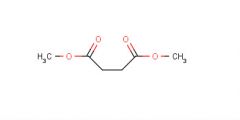
What is this molecule's name?
What is it's purpose? |
|
|
Dimethyl fumerate
Product |
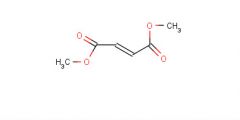
What is this molecule's name?
What is it's purpose? |
|
|
Morpholine
Nucleophile |
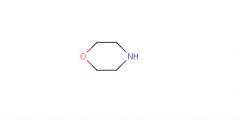
What is this molecule's name?
What is it's purpose? |
|
|
ZnCl2
Lewis Acid |

What is this molecule's name?
What is it's purpose? |
|
|
Methanol
Reaction Solvent |

What is this molecule's name?
What is it's purpose? |
|
|
Diethyl ether
Used in extractant |
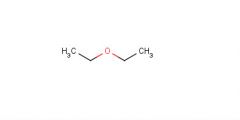
What is this molecule's name?
What is it's purpose? |

