![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
General formula for saturated hydrocarbon
|

saturated hydrocarbon
|
|
|
Give the relative energies for all Newman projections of an alkance and state their abs and relative maxs and mins
|
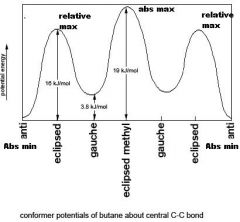
Relative energies
|
|
|
Which is more stable axial or equatorial bond?
Where should large groups go, equatorial or axial? |

equatorial bond energy is lower because not as crowded
Large groups are more stable of equatorial |
|
|
Why are cyclopropane and cyclobutane less stable than cyclo pentanes are heavier?
|
Because the But/propane has ring strain forcing their sp3 hybridized bonds into 90/60 degree angles, far from the ideal 109
|
|
|
What is the formula for hydrocarbon combustion?
What does it require? |
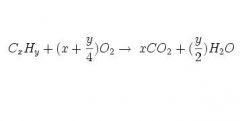
High temp and available oxygen
|
|
|
Describe the 5 steps of free-radical halogenation of alkanes
|

free-radical halogenation of alkanes
|
|
|
Sp3 to Sp2
Forms both R and S depending on whether halogen came from top or bottom |
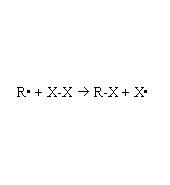
Describe orbital change for free radical halogenation of alkane and orientation of R-X
|
|
|
Describe regioselection of free radical halogenation of alkane for bromination and chloronation
Which is faster, why? |

regioselection
Chloronation is faster because it is highly exoterminc (-H) for both propogation steps. Bromination is exothermic only in 1 |
|
|
Why is ortho-nitrophenol boil faster than para-nitrophenol
|
Para-nitrophenol has the OH at the para end, allows more space for intermolecular interaction
Ortho has intramol. interaction with nitro group, less H bonding means lower bp |
|
|
Which is more acidic: para nitrophenol or para methoxyphenol?
|
Para-nitro phenol
Nirto para is E withdrawing and stablaizes the better because the resonance structure keeps the charges close together (close seperation of charges is better if they exist at all) |
|
|
Describe the Sn2 rxn of butanol with hydrobromide
what is the rxn rate? Does a carbocation form? Is there regioselection? |
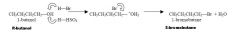
The strong acid causes -OH, a poor leaving group, to become protonated, -OH2 which is a good leaving group (H20)
Rxn rate = k [nuc][elec] = k [Br-][R-OH] (depends on both hence sn"2" A carbocation never forms There is complete stereochemical inversion, R->S |
|
|
What is the reactivity order of R-OH's in sn2? (ter, sec, and prim)
Why? |
1>2>>3
Due to steric hindress to backside attack |
|
|
What kind of solvent is favored by sn2 rxn's?
|
Polar, non-hydrogen bonding solvents
|
|
|
Describe the sn1 reaction of t-butanol with hydrobromide
What is the rate? Is there a carbocation formed? Is there regioselectivity? |

Sn1
|
|
|
What type of solvent is favored by Sn1 and why?
|
Hydrogen bonding solvents because they stabilize the carbocation
|
|
|
What type of R-OH (3,2,1) favors Sn1?
Why? |
3>2>1
because the carbocation is most stable on 3 |
|
|
Resonance structures cannot be drawn through __________
|
Truly sp3 carbons
|
|
|
In the rotation around a sigma bond, between what stages is the greatest change in potential energy?
What point is the greatest PE? |
Between gauche staggered -> gauche eclipsed -> gauche staggered
Gauche eclipsed is the most unstable and thus highest PE |
|
|
Why do cyclobutanes and cyclopropanes undergo hydrogenation but cyclopentanes/hexanes don't?
|
Cyclobu/propanes have ring strain which make them unstable and suseptible to hydrogenation.
No ring stain (or very little for pentane) doesn't undergo hydrogenation |
|
|
Why does a 1,2 hydride or methide shift occur?
|
To stabilize a carbocation.
|
|
|
Alkene + alkyl halide
|
More substituted carbocation forms (2nd will form before primary)
Then rearrgangement (to tert)and then/ or halide addition |
|
|
Alkene + peroxide + alkyl halide
|
Peroxide undergoes homolytic cleavage R-O. + R-O.
Makes halide radical R-O. + H-Br > R-OH + B. Anti-Markovnikov Rule, halide radical adds to lowest substituted C (usually primary, but 2nd can) |
|
|
Alkene + H2SO4 + H2O
|
Forms carbocation with rearrangements, so -OH adds to most substituted C
|
|
|
Is there a carbon substitution preference for E1 vs E2 reactions?
|
E1 will work with 3 and some 2ndary Carbons BUT NOT PRIMARY
E2 is 3>2>1 |
|
|
Is there a product preference for E1 and E2 reactions?
|
E1 forms more substituted product and E2 forms has anti arrangement
|
|
|
How can you use pKa to determine a good leaving group?
|
The lower a pKA is of the conjugate acid, the better leaving group it is
|

