![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
57 Cards in this Set
- Front
- Back
|
What is Pharmacolinetics of Inhalational Anesthetics?
|
quantitative study of:
absorption (uptake) from alveoli into pulmonary capillary blood distribution in the body metabolism, and excretion - principally via the lungs |
|
|
What determines the partial pressure of anesthetic in the blood and, ultimately, in the brain?
|
Alveolar Partial Pressure
|
|
|
What are three factors that affect anesthetic uptake?
|
solubility in the blood
alveolar blood flow difference in partial pressure between alveolar gas and venous blood |
|
|
FGF (fresh gas flow) is determined by ________ and __________ settings.
|
vaporizer, flowmeter
|
|
|
FI (inspired gas concentration) is determined by ________,_________, ___________ .
|
FGF rate; breathing-circuit volume; circuit absorption
|
|
|
FA (alveolar gas concentration) is determined by _________, __________, _______, _______________.
|
uptake; vetilation; concentration effect; second gas effect.
|
|
|
Fa (arterial gas concentration) is affected by ____________ mismatching.
|
ventilation/perfusion
|
|
|
What is an indirect measurement of anesthetic partial pressure in the brain?
|
Alveolar partial pressure (PA)
PA - mirrors brain level |
|
|
What is concentration effect?
|
high, initial inhaled partial pressure of agent,offsets uptake into the blood and accelerates rate of induction of anesthesia.
|
|
|
What is second gas effect?
|
the concentration effect of one gas upon another. Usually refers to nitrous oxide combined with an inhalation agent.
N2O has a low blood solubility, is rapidly absorbed from the alveili into pulmonary circulation to significantly alter concentration of remaining gasses in alveoli. |
|
|
Factors affecting Alveolar concentration
|
Inhaled Partial Pressure of agent
Uptake of inhaled agents into the pulmonary circulation Alveolar ventilation Characteristics of delivery system - volume of anesthetic breathing system acts as a buffer to attain high PA. High gas flows negate this buffer effect. "Wash In" |
|
|
What determines the Uptake of inhaled anesthetics from Alveoli to Pulmonary Capillary Blood?
|
Solubility of the anesthetics in blood and body tissue.
Cardiac Output Alveolar to Venous partial pressure differences (A-vD) |
|
|
Solubility describes the affinity of the gas for a medium such as blood or fat tissue and is expressed as ___________ .
|
Partition Coefficients
|
|
|
What is the Ostwald Solubility Coefficient?
|
Volume (ml) of gas dissolved per ml of liquid (blood) at ambient temperature and pressure (assuming body temp 37 C, and atmospheric pressure 760 mm Hg)
|
|
|
Partition Coefficients are TEMPERATURE dependent. Solubility of a gas in a liquid is decreased when the temperature of the liquid _______________.
|
increased.
|
|
|
Alveolar partial pressure is an indirect measurement of partial pressure in the _________.
|
brain
|
|
|
A gas that is highly soluble (high uptake) causes _________ alveolar concentration, therefore a __________ induction
|
lower; slower
|
|
|
Low solubility (not easily dissolved in blood, less uptake) = higher alveolar concentration means __________induction and _________ emergence.
|
faster; faster
|
|
|
Takes longer to build up partial pressure in the blood and brain if agent has ________solubility, which means _________ alveolar concentration.
|
higher; lower
|
|
|
Higher Blood:Gas Partition coefficient, _____ anesthetic's solubility so greater uptake in pulmonary circulation. This means _______ alveolar pressures and ______ induction and emergence.
|
higher; lower; slower
|
|
|
Lower Blood:Gas coefficient - less solubility in the blood and less uptake into pulmonary circulation. This means ______ alveolar partial pressure and ______induction and emergence.
|
higher; faster
|
|
|
What is Blood:Gas solubility for:
Desflurane _____ N20 ___________ Sevo ___________ Iso _________ |
0.42
0.47 0.69 1.43 (remember from smallest to greatest) |
|
|
Brain:Blood Solubility of
N2O ________ Des_______ Iso ________ Sevo_________ |
0.5
1.3 1.6 1.7 |
|
|
Ultimate goal of anesthesia is adequate _______ level to have desired effect.
|
Brain
|
|
|
In the absence of pulmonary shunt, Alveolar blood flow is equal to _______
|
Cardiac Output (CO)
|
|
|
Increase in CO will _________ in anesthesia uptake and will _________ in alveolar concentration. Induction will be ______.
|
increase; decrease; delayed
|
|
|
Decrease in CO will ___________ in anesthesia uptake and _________ in alveolar concentration. Induction will be ______
|
decrease; increase; fast
|
|
|
In the absence of an intra-cardiac or intrapulmonary shunt, PA and Pa of inhaled anesthetics are essentially ________
|
identical
|
|
|
Right-to-left shunt, dilutes effect on the partial pressure of anesthetic in blood coming from ventilated alveoli, = ______ in arterial concentration, _______ of induction
|
decrease; slowing
|
|
|
What reflects tissue uptake of the inhaled anesthetic. This gradient depends upon tissue uptake.
|
Alveolar-to-venous (A-vD) partial pressure differences
|
|
|
What are three things that determine transfer of anesthetic agent from the blood to the tissues?
|
1) Tissue solubility (tissue/blood partition coeffecient)
2) Tissue blood flow 3) difference in arterial and tissue partial pressure of the anesthetic agent |
|
|
What determines the uptake of inhaled anesthetics from Alveoli to Pulmonary Capillary Blood?
|
1) solubility of the anesthetic in blood and body tissues
2) Cardiac Output 3) Alveilar to venous partial pressure differences (A-vD) |
|
|
What is the Context - Sensitive Half Time?
|
Computer simulations determining the time needed for a 50% decrease in anesthetic concentration of volatile agents. Initial phase of elimination. Primary a function of alveolar ventilation.
|
|
|
What factors speed up induction and recovery?
|
high fresh gas flows
low anesthetic - circuit volume decreased solubility of agent increased ventilation |
|
|
What is Diffusion Hypoxia and how to prevent it?
|
occurs when inhalation of Nitrous Oxide is discontinued abruptly. Causes reversal of partial pressure gradients. N2O leaves blood and enter alveoli.
Prevented by adm 100% O2 for 5-10 min after discontinuing N2O |
|
|
Potency is related to _________
|
lipid solubility (gets through lipid membrane faster)
|
|
|
General anesthetics are "dangerous" with therapeutic indixes _________
|
2-4 (can produce circulatory failure)
|
|
|
Although brain is site of action, anesthetic potency is measured on the basis of ___________ that produces immobility in 50% of patients exposed to painful stimuli.
|
alveolar gas concentration
|
|
|
Awareness or recall is highly unlikely at the MAC levels of________
|
1.2-1.3 MAC
|
|
|
MAC is inversely related to ________
|
potency
|
|
|
Define Potency.........
|
a content of drug required to produce a desired specific effect. A highly potent drug gives a large response at low concentrations. A lower potency drug evokes a small response at low concentrations.
|
|
|
Define Efficacy............
|
relationship between occupancy of the receptor and ability to start a response
|
|
|
Define Affinity .............
|
the ability of the drug to bind to the receptors.
|
|
|
MOA of Inhaled Anesthetics and what is Meyer - Overton rule.....
|
MOA is poorly understood
Meyer-Overton rule - proposes that all inhalation agents share a common MOA at the molecular level; anesthetic potency of inhalation correlates directly with their lipid solubility Meyer-Overton rule also postulates that the number of molecules dissolved in the lipid cell membrane, and not the type of inhalation causes anesthesia. Evidence that general anesthetics may act by binding to only a small number of targets in the CNS. Voltage-Gated Channels - unlikely to play a substantial role production of anesthetic state. Ligand-Gated Ion Channels - may enhace affinity of GABA for its receptor-relevant to production of anesthetic-induced immobility |
|
|
Oldest anesthetic agent sill in use today_________
|
Nitrous Oxide
|
|
|
Gas that is ONLY inorganic anesthetic gas in clinical use is_________
|
Nitrous Oxide
|
|
|
Only inhalational anesthetic used today that is not a vapor and is a true gas by definition is_________
|
Nitrous Oxide
|
|
|
What gas is this?
MOA - unknown Prominent analgesia BUT minimal muscle relaxation Causes PONV. MAC 104% Can lease blood to enter an air-filled cavity 34 times faster than nitrogen. Minimal if any effects on liver, kidney or GI tract |
Nitrous Oxide
N=N=O |
|
|
What gas is this?
Flammable, irritating vapor, slow onset and prolonged recovery. Exposion hazard. |
Diethyl Ether
|
|
|
What gas is this?
Halogenated alkane (Alkane contain only H and C atoms bonded by single bond) Halogenated - replacement of one or more hydrogen atoms by a halogen (fluorine chlorine bromine or iodine) THYMOL - is the preservative. Stays in vaporizers after and can cause malfunction. Non flammable, relatively POTENT. Unpredictible CHEMICAL HEPATITIS Significant Myocardial Depression & irritability. |
Halothane
|
|
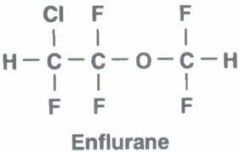
|
halogenated methyl ethyl ether
Less arrhythmia, N/V, post-op shivering than Halothane High potency, rapid onset and recovery, muscle relaxation Concern in pts w/SEIZURE Disorder Used in adults not so much on peds Metabolite FLUORIDE usually does not reach levels required for renal toxicity |
|
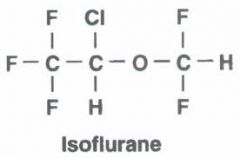
|
halogenated methyl ethyl ether
Chemical ISOMER of Enflurane, but has different chemical properties Rapid, smooth depth of anesthesia with limited effects on pulse or respiration NO hepatic/renal toxicity CBF and ICP reaily controlled Arrhythmia uncommon Skeletal muscle relaxation |
|
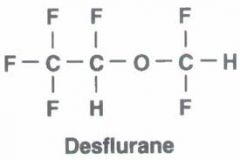
|
fluorinated methyl etheyl ether. Differs from Iso only by substitution of a fluorine atom for a Cl atom.
High vapor pressure, BOILS at room temp. Heated and pressurized and requires electrical power. Limit solubility - RAPID ONSET & RECOVERY. Little renal/hepatic metabolism Airway irritant, NOT FOR INDUCTION |
|
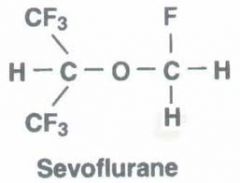
|
Fluorinated methyl isopropyl ether
Vapor pressure resembles Halothane & Iso LOW blood solubility, HIGH potency 3-5% metabolized, releasing more Floride. Does not cause hepatitis Degradation in CO2 absorber. Can form Compound A. Keep fresh gas flows above 1L/min |
|
|
********FACTS********
Halogenation reduces flammability Fluorination reduces solubility Fluorination decrease potency Substitution of Cl for F (Iso vs. Des) decreases solubility decreases potency increase Vapor pressure and decreases boiling point |
************more FACTS**********
All inhaled anesthetics liquid at 20 C Vapor Pressure: Pressure exerted by the molecules of the vapor phase at equilibrium of molecules moving in and out of liquid phase Vapor Pressure dependent on temp and physical characteristics of liquid, independent of atmospheric pressure Increased temp increases vapor pressure Boiling Point: Temp at which vapor pressure equals atmosphetic pressure |
|
|
Variable Bypass Vaporizer (Datex Ohmeda Tec 4,5,7 or North American Drager Vaporizer 19.n and 20.n
|
Halothane
Enflurane Isoflurane Sevo |
|
|
Tec 6 Vaporizer: Electroniclly heated, thermostatically controlled, pressurized, electromechanically coupled, dual circuit gas-vapor blender
|
Desflurane
|

