![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
Sulponamides - basis |
•Analogue of para-aminobenzoic acid (PABA)•Essential for folic acid synthesis •Mammalian cells use preformed folic acid and cannot synthesize •Bacteria synthesize and cannot take up folic acid preformed |
|
|
Sulphonamides - spectrum of activity |
•Broad spectrum bacteristatic |
|
|
Sulphonamides - mechanism of activity ? |
•Block stage in folate synthesis (inhibits dihydropteroate synthase) •Sulphonamides competitive binding •Folate depletion – failure to produce purine nucleotides and thymidine |
|
|
Three enzyme in folate synthesis 1) DHPS 2) DHFS 3) DHFR enzyme 1 and three can be inhibited via ? |
1) Sulphonomides 2) - 3) Trimethoprim |
|
|
Sulphonamides -why do they take a long time to work? |
•need folate depletion which can take a few generations. |
|
|
Sulphonamides - commonly used in combination with |
Trimethoprim |
|
|
Sulphonamides - side effects |
•Stevens-Johnson Syndrome - inflammation of the skin andmucous membranes. •Eg eyes, digestive system, lungsand respiratory system (can be fatal) |
|
|
Trimethoprim - mechanism of action ? |
•Inhibit dihydrofolate reductase – later stage infolate synthesis •Much higher affinity for the bacterial enzyme than the mammalian |
|
|
Trimethoprim - spectrum of activity ? |
•Broad spectrum bacteristatic |
|
|
Trimethoprim - side effects ? |
rare |
|
|
Trimethoprim witha Sulphonamide - mechanism? -e.g. co-trimoxazole to treat ...... -e.g. UTI |
•to act at 2 locations in the same pathway (folate systesis pathway) •but probably as effective and less toxic alone |
|
|
Co-trimoxazole- (Trimethoprim + sulphamethoxazole) - demonstrate ............ |
•synergy |
|
|
(Trimethoprim + sulphamethoxazole) spectrum of activity ? |
•Rarely used in UK, but sometimes when resistance to beta lactams andciprofloxacin have occured + Pneumocystis carinii, fungal pneumonia, AIDS -neither are active against Pseudomonas |
|
|
Inhibitorsof nucleic acid synthesis -mechanism of action |
•Interfere with DNA and RNA functions •(May be due to different pathways) •Different structure of bacterial genome •Enzymes not possessed by mammalian cells •Important due to universal nature of the target•Not PeptiDoGlycan or “different ribosomes” |
|
|
Quinolones - names/types |
- Nalidixic acid - Norfloxacin - Ciprofloxin |
|
|
Quinolones - spectrum of activity? |
•Gram negatives – except pseudomonas •Used exclusively for UTIs |
|
|
Quinolones - Well absorbed ..........., excerted in ......... |
• orally , urine |
|
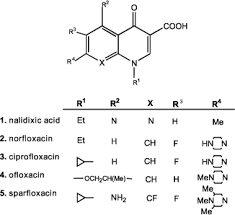
2 alterations improved activity: |
•A F in C6( flumequine) •Piperazineat C7 (pipemicidic acid) •C7 can very but F a constant •Fluoroquinolones |
|
|
Fluoroquinolones - spectrum of activity ? |
•Gram positive and Gram negative including Pseudomonas •Largely replaced quinolones which showed little G+ve activity |
|
|
Fluoroquinolones - names/types ? |
•Ciprofloxacin, moxifloxacin |
|
|
Fluoroquinolones - ROA? |
•Oral administration |
|
|
Fluoroquinolones - well distributed through tissue & concentrated in mammalian cells , thus good for treating ? |
intracellular pathogens : •(chlamydia, legionella, some mycobacteria) •Antibiotic of choice for typhoid fever |
|
|
Fluoroquinolones - resistance ? |
•becoming a problem ( especially when used in animal husbandry) |
|
|
Fluoroquinolones - mechnism of action ? |
•Gram negatives – target DNA topoisomerase type II (gyrase) with type IVas a secondary target. •Gram positives – opposite |
|
|
DNAgyrase (type II) does what ? |
•supercoiling |
|
|
Topioisomerase TypeIV does what? |
replication,recombination and repair |
|
|
Fluoroquinolones - resistance mechanisms? |
•Resistance usually due to chromosomal mutation •Modified structure of type II and IV •Decrease membrane permeability •Over-reactive efflux pumps actively removing drug |
|
|
Fluoroquinolones - side effects |
•Side effects – rashes, gastrointestinal and non specific neurologicalsymptoms •Not recommended for children or pregnant •Some have had to be withdrawn due to unexpected toxicity |
|
|
Nitrofurans- spectrum of activity ? (N.B: More active in acidic conditions) |
•Active against Gram negative and positive urinary pathogens only – exceptProteus and Pseudomonas •Oral dosage followed by rapid excretion in the urine, also inactivatedin tissues (NOT intra-cellulars!) |
|
|
Nitrofurans - resistance ? |
•Resistance uncommon |
|
|
Nitrofurans - side effects |
• nausea |
|
|
Nitrofurans - mechanism of action ? |
uncertain ? -•Possible the nitro compound is reduced by the organism – affects DNA |
|
|
Rifampicin - mechanism of action |
•Binds to Beta subunit of DNA-dependant RNA polymerase – inhibits mRNAsynthesis |
|
|
Rifampicin - ROA |
well absorbed orally |
|
|
Rifampicin - spectrum of activity ? |
•Active against Myco tuberculosis and leprae, staphylococci, legionella |
|
|
Rifampicin - resistance? |
occursrapidly – used in combination •Unfortunately resistance can occur due to mutations in this subunit which is encoded on thechromosome •So often used with other agents •But useful for determining frequency of point mutations is a species |
|
|
Rifampicin - used to treat |
-TB,leprosy, Legionnaires disease and staphylococcal infections (never alone) - Prophylaxis against meningococcal and Haemophillus influenzae type b disease |
|
|
Rifampicin - side effects? |
•hepatotoxicity can occur •Interfere with other drugs handled by the liver – oral contraceptives •Pigmentation can lead to red bodily secretions |
|
|
Abrief look at Mycobacteria •Issues: waxy coat, slow growing, tendency to survive with macrophages . use what drugs? |
•General: include Rifampicin and Streptomycin. •Also some macrolides and fluoroquinolones -Specific :Isoniazid, ethambutol, cycloserine ( if first line fails) |
|
|
A brief look at Mycobacteria •Issues: waxy coat, slow growing, tendency to survive with macrophages . WHY? do we use these drugs |
•Hard to study due to growth rate. •Mode of actions, inhibit cell wall formation? Isoniazid..mycolic acids •Ethambutol … arabinoalacatan inhibitory •target DNA topoisomerase type II (gyrase) • inhibition of bacterial protein biosynthesis |
|
|
Metronidazole- spectrum of activity ? |
•Active against anaerobes |
|
|
Metronidazole - reistance ? |
•Resistance uncommon |
|
|
Metronidazole - mode of action |
uncertain– possible reduction product formed at low redox potential induce DNA strandbreakage |
|
|
Metronidazole - side effects with alcohol |
•nausea and vomiting, vasodilatation, flushing, headaches, palpitations, tachycardia |
|
|
Metronidazole - side effects without alcohol |
•Damages DNA , genotoxic and tumourogenic,while no evidence, avoid use in pregnancy |
|
|
Membraneactive agents - names/types |
•Polymixins •PolymixinB and Colisitin (E) •Polypeptides with long hydrophobic tail |
|
|
Membrane active agents- mechanism of activity ? |
•Bind to cell membrane causing leakage of cytoplasm |
|
|
Membrane active agents- harmful? |
•Considerable toxicity |
|
|
Membrane active agents- spectrum of activity ? |
•Active against Gram negatives except Proteus – including inactiveorganisms |
|
|
Membrane active agents- ROA |
•Systemic use limited, im injection •Used topically |
|
|
Polymixin B and Colisitin can be used to eradicate ............... infection in patients groups? |
Pseudomonas infections in CF patients |

