![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
Methane |
CH4 |
|
|
Ethane |
C2H6 |
|
|
Propane |
C3H8 |
|
|
Butane |
C4H10 |
|
|
Acetylene |
C2H2 |
|
|
Calcium Carbide |
CaC2 |
|
|
Ozone |
O3 |
|
|
Ammonium |
NH4+ |
|
|
2,0 |
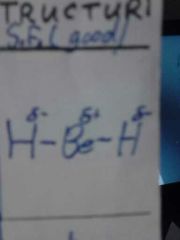
Linear 180° MF ie. BeH2 |
|
|
3,0 |
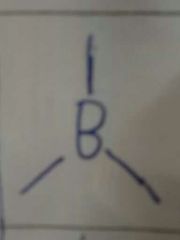
Trigonal Planar 120° MF ie. BH3 |
|
|
4,0 |

Tetrahedral 109° CH4 |
|
|
3,1 |
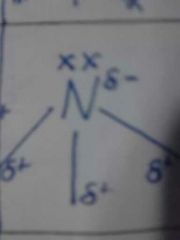
Trigonal Pyramidal 107° NH3 |
|
|
2,2 |
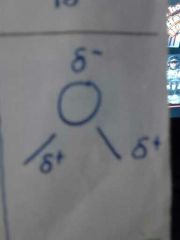
Bent 104.5° H2O |
|
|
HI |
Hydroiodic acid |
|
|
Sulfate |
SO4 2- |
|
|
Nitrate |
NO3 - |
|
|
Chlorate |
ClO3 - |
|
|
Carbonate |
CO3 2- |
|
|
Phosphate |
PO4 3- |
|
|
Acetate |
CH3COO- |
|
|
Intermolecular Forces |
Ionic Forces=Ionic Hydrogen Bonds=Polar and H bonded to N, O, and F Dipole-Dipole=Polar London Dispersion Forces= Non-polar and everything else |
|
|
Ionic Bonds |
Solids Hard Very High BP/MP Called molten when melted Dissociates in water |
|
|
Hydrogen Bonds |
Liquids High BP/MP Dont conduct Dissolves in water |
|
|
Dipole-dipole |
Liquids Medium BP/MP Don't conduct Dissolves in polar |
|
|
LDF |
Usually gases Low BP/MP Like dissolves like Don't conduct |

