![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
304 Cards in this Set
- Front
- Back
|
What are the 3 spaces in the brain? Which is an actual space and which is a potential space?
How are hemorrhages caused in these spaces? |
Epidural space - between skull and dura
• Middle meningeal <b>artery</b> • Bleed = biconvex shape, doesn't cross suture lines Subdural space - between dura and arachnoid • Site of bridging <b>veins</b> • Bleeding = crescent shaped, crosses suture lines Subarachnoid space - ACTUAL space between arachnoid, pia • Houses CSF • Subarachnoid hemorrhage = "worst headache of my life" • <i>May become clouded in meningitis</i> (first, <b>worst</b>, cursed) |
|
|
What are meningiomas?
|
Tumors of arachnoid cap cells
• Benign, slow growing, well-circumscribed • Removed surgically |
|
|
Where is CSF produced?
|
Choroid plexus (lateral, 3rd, 4th ventricles)
|
|
|
How does CSF flow?
|
Lateral ventricles →
Foramina of Monroe → 3rd ventricle → Cerebral aqueduct → 4th ventricle → Foramen of Magendie (medial) Foramina of Luschka (lateral, 2x) → Subarachnoid space → Arachnoid granulations/villi |
|
|
Where does a lumbar puncture occur? What are some disease it is used to diagnose?
|
L3/L4 interspace
• Below the end of the spinal cord (L1, L2) • Cauda equina roots will not be damaged Diseases: • Oligoclonal bands in multiple sclerosis • Blood in subarachnoid hemorrhage • PMNs in meningitis |
|
|
What are 2 problems with flow of CSF?
|
1. Hydrocephalus- Obstructive (non-communicating)
• Congenital stenosis of the cerebral acqueduct (3rd, lateral) • Arnold-Chiari malformation - brainstem gets pulled through forman magnum (4th, 3rd, both lateral) 2. Normal pressure (communicating) - in elderly • ↓ resorption of CSF at arachnoid granulations (e.g. due to scarring) |
|
|
What are the clinically important branches of the internal carotid a.?
|
1. Ophthalmic - monocular blindness
2. Middle cerebral (MCA) - lateral surface of frontal, parietal, temporal (contralateral motor and sensory deficits in <b>face/arm > leg</b> + aphasia in dominant (left) hemisphere) 3. Anterior cerebral (ACA) - medial surface of frontal, parietal (contralateral motor and sensory deficits in <b>leg > arm and face</b>) 4. Posterior communicating artery (aneurysms) 5. Anterior communicating artery (aneurysms) <a href="http://www.bcnlp.ac.th/Anatomy/page/apichat/cardio-vascular/picture/brain-cir2.jpg">Cerebral Circulation Diagram</a> |
|
|
What are the perforating or ganglionic arteries?
|
Supply deep portions of cerebral hemispheres in diencephalon
If internal capsule (contralateral motor and sensory deficits in <b>face = arm = leg</b> → hemiplegia) |
|
|
Describe the vertebral (basilar and posterior) circulation of the brain.
|
1. Vertebral arteries
• Anterior, posterior spinal arteries • PICA - lateral medullary syndrome (Wallenberg's) 2. Basilar artery • AICA • Internal auditory a. - vertigo, ipsilateral deafness 3. Posterior cerebral artery (PCA) • Midbrain (ventral, Weber's syndrome) • Diencephalon (thalamic syndrome of Dejerine-Roussy) • Medial, inferior surfaces of temporal, occipital lobes (hemianopsia) 4. Circle of Willis • Connects anterior, posterior circulations • Aneurysms → subarachnoid hemorrhagic strokes ***Especially at branch points, e.g. communicating arteries |
|
|
Describe spinal cord circulation.
|
1. Anterior spinal artery
• Biggest • Anterior 2/3 of spinal cord (ventral horn, anterolateral white columns) • Stroke = anterior cord syndrome *Medial Medullary Syndrome 2. Posterior spinal arteries • Right, left • Dorsal white column, dorsal horn |
|
|
What are the clinically important pathways of the spinal cord? (3)
|
1. Dorsal column
• Touch, pressure, vibration, proprioception • Fasciculus gracilis (LE), fasciculus cuneatus (UE) • IPSILATERAL 2. Spinothalamic tract • Pain, temperature • CONTRALATERALLY 3. Lateral corticospinal tract • Motor commands from CONTRALATERAL cortex *UMN damage = contralateral damage *LMN damage = ipsilateral damage (decussation at pyramids) |
|
|
Which order of neurons decussate?
|
2nd order
|
|
|
How are UMN and LMN lesions different?
|
LMN - lowered
• Weakness, atrophy, ↓ tone, ↓ reflexes, ↓ Babinski UMN - up • ↑ tone, fasciculations, ↑ reflexes, clonus, ↑ Babinski, spastic paralysis |
|
|
What happens with root lesions of spinal cord?
|
Ipsilateral
1. Dorsal root - paresthesia → pain → anesthesia 2. Ventral root - LMN weakness, flaccid paralysis ↓ reflexes in both e.g. L5/S1 disc prolapse = S1 dorsal + ventral root compression • Sciatica, pain down lateral side of foot (S1 dermatome) • Foot eversion, plantar flexion weakness (S1 myotome) • Loss of ankle jerk reflex |
|
|
What is complete cord transection?
|
Trauma, inflammation (e.g. transverse myelitis)
Bilateral signs Anesthesia below the level of lesion UMN injury: Initial flaccid paralysis from spinal shock → spastic paralysis (UMN) |
|
|
What are some incomplete lesions of the spinal cord?
|
1. Anterior cord syndrome - stroke of anterior spinal artery
• Only dorsal columns intact = touch, pressure, vibration, proprioception NORMAL • Bilateral UMN lesion below injury (CST) • Pain, temperature sensation lost bilaterally below injury (STT) 2. Hemisection (Brown-Sequard) • Ipsilateral loss of touch, pressure, proprioception AT AND BELOW lesion • Ipsilateral UMN signs AT AND BELOW lesion • Contralateral loss of pain and temperature, 1-2 segments BELOW lesion (axons ascend as they cross) |
|
|
What is central cord syndrome?
|
Trauma - usually neck (e.g. whiplash), Arnold-Chiari malformation
Syringomyelia - fluid-filled cavity (syrinx) in center of spinal cord • Pain/temperature cross in center → BILATERAL "band" loss of pain and temperature - normal above and below May result in LMN symptoms if it extends into ventral horn |
|
|
Motor nuclei are ____ to the sulcus limitans. Sensory nuclei are ____.
|
Motor - medial
Sensory - lateral *Medial brainstem lesions = motor, lateral = sensory |
|
|
What are the 4 clinically important spinal cord/brainstem pathways?
|
ALL CONTRALATERAL
1. Medial lemniscus (DC-ML) - touch, pressure, vibration, proprioception 2. Spinothalamic - pain and temperature 3. Corticospinal tract - movement commands (contralateral side, hasn't crossed yet) 4. Corticobulbar tract - movement commands to contralateral lower face |
|
|
Describe the 2 possible lesions of the facial nerve.
|
1. UMN lesions - stroke of internal capsule
• Contralateral <b>lower face</b> paralysis *May damage corticospinal tract as well = contralateral hemiplegia 2. LMN lesions - Bell's palsy • Ipsilateral flaccid paralysis, ↓ reflexes |
|
|
The tongue deviates ____ the lesioned side. The uvual deviates ____ the lesioned side.
|
Tongue - towards
Uvula - away Corticobulbar fibers crossed to genioglossus, but uncrossed to SCM and trapezius. |
|
|
Describe some characteristics of brainstem lesions in general.
|
Damage to:
1. CNs 2. Ascending spinal cord pathways (medial lemniscus, spinothalamic) 3. Descending (corticospinal) = Crossed/Alternating syndromes *Ipsilateral CN deficits (LMN flaccid paralysis, sensory loss) *Contralateral hemiplegia (UMN spastic paralysis, sensory loss) |
|
|
What is medial medullary syndrome?
|
Alternating Hypoglossal Hemiplegia
*occlusion of vertebral, anterior spinal a. • CST - contralateral hemiparesis • DC-ML - contralateral sensory deficits (PPTV) • CBT - ipsilateral flaccid paralysis of tongue, difficulty speaking/swallowing *Tongue deviates toward lesion side |
|
|
What is lateral medullary syndrome?
|
Wallenberg's syndrome - alternating hemianesthesia
*occlusion of PICA • STT - contralateral loss of pain and temperature from body • TTT - ipsilateral loss of pain and temperature from face • CBT - dysphagia, dysphonia, dyspnea - ipsilateral paralysis of larynx, pharynx, soft palate (nucluus ambiguus and IX, X) → uvula deviates to opposite side (LMN lesion) |
|
|
What is ventral syndrome of the midbrain?
|
Weber's syndrome - alternating occulomotor hemiplegia
*occlusion of branch of posterior cerebral artery • CST, CBT - contralateral hemiplegia and lower facial paralysis • CBT - ipsilateral oculomotor n. palsy |
|
|
What happens in the thalamus?
|
Subcortical structures synapse in the thalamus before going on to the cortex
= RELAY for ascending sensory information |
|
|
What is the lateral geniculate nucleus (LGN)? Medial (MGN)?
|
Lateral = Light (visual)
• From optic tract fibers of retina to primary visual cortex Medial = Music (auditory) • From inferior colliculus to primary auditory cortext (Heschl's gyrus) **Found in the metathalamus |
|
|
What are the ventral nuclei of the lateral group of thalamus?
|
Ventral posterior - relay for somatic sensation
1. VPL - body • DC-ML • Spinothalamic 2. VPM - head • Trigeminal lemniscus • Trigeminothalamic Ventral anterior (VA) - motor from globus pallidus Ventral lateral (VL) - motor from dentate nucleus of cerebellum |
|
|
What are the "other" nuclei of the thalamus?
|
1. Dorsal tier nuclei - part of lateral nuclear group
• Integration of somatic, visual, auditory sensations 2. Medial nuclear group • Affective behavior 3. Anterior nuclear group • Limbic system |
|
|
What is the thalamic syndrome of Dejerine-Roussy?
|
Occlusion of posterior cerebral artery supplying VPL, VPM
• Initially: contralateral hemisensory loss in head (VPM), body (VPL) → DC-ML, STT, TTT, TL • Then: 1. Dysesthesia - disagreeable sensation with ordinary stimuli 2. Spontaneous, intractable thalamic pain 3. Emotional instability |
|
|
What are the subdivisions of the cerebellum?
|
1. Vestibulocerebellum
• <b>Flocculonodular lobe</b>, vermis, fastigial nucei • Balance - eye movements, muscle tone • Vestibulospinal, reticulospinal, ventral corticospinal 2. Spinocerebellum • <b>Anterior lobe</b>, vermal/intermediate zones of posterior lobe, fastigial/interposed nuclei • Coordination of posture, locomotion, limb movements in response to proprioceptive input 3. Cerebrocerebellum • <b>Posterior lobe</b> • Input/output to opposite cerebral cortex • Motor planning, initiation, timing, cognitive functions, learned/skilled movements |
|
|
Unilateral lesions of the cerebellum result in ___lateral deficits
|
IPSILATERAL
|
|
|
Trunk muscles are represented _____ in the vermal, intermediate zones of the cerebellum, while limb muscles are represented _____ in the hemispheres.
|
Trunk - medially
Limb - laterally |
|
|
What are the deficits with midline vermal lesions?
|
Truncal ataxia - wide-based gait
1. Positive Romberg sign 2. Falling toward lesion side 3. Nystagmus |
|
|
What are the deficits with lateral hemisphere lesions?
|
Limb ataxia
• ↓ tone, ↓ reflexes • Trouble with movement initiation • Tremor • Decomposition of movement • Dysmetria (over/undershoot) • Dysdiadochokinesis (timing, sequencing of alternating movements) • Dysarthria (abnormal articulation) |
|
|
Basal ganglia
|

Blue = inhibitory
Red = excitatory |
|
|
What is the result of a stroke in the subthalamic nucleus (STN)?
|
Messes up the "stop" pathway = ↑ excitation
= hyperkinetic involuntary violent flinging movements = Hemiballismus |
|
|
What is Parkinson's?
|
Degeneration of SNc
= ↑ indirect pathway facilitation = ↓ excitation of motor cortex = rigidity, dystonia, akinesia, bradykinesia |
|
|
What do Huntington's and Wilson's diseases do?
|
First:
• Affect indirect pathway = ↑ excitation to cortex • Chorea, athetosis involuntary movements Later: • Affect direct pathway • ↓ excitation = looks like Parkinson's (rigidity, dystonia, etc.) |
|
|
What are the primary sensory areas of the cortex? What happens with damage to each?
|
1. Primary somatic sensory cortex (S1)
• Brodmann areas 3,1,2 in postcentral gyrus • Damage = impairment of finer aspects of sensation; serious deficit in proprioception 2. Primary visual cortex (V1 or striate area) • Occipital lobe, area 17 • Damage = hemianopsia, loss of vision in contralateral half of each visual field 3. Primary auditory cortex (A1) • Heschl's gyrus • Damage = some difficulty localizing contralateral sounds, subtle hearing loss contralaterally |
|
|
What are the sensory association areas?
|
1. Somatic sensory assocation cortex
• Posterior parietal cortex, superior parietal lobule • Right-sided damage = contralateral neglect • Left-sided damage = apraxia (inability to perform learned movements w/o paralysis) 2. Visual association areas (extrastriate cortex) • Occipital lobe • Ventral = WHAT → damage = agnosia, prosopagnosia • Dorsal = WHERE → damage = visual neglect contralaterally (bump into stuff!) 3. Auditory association area (A2) • Right side = ID familiar sounds and music → auditory agnosia • Left side = Wernicke's area = understanding speech → receptive or sensory aphasia |
|
|
What are the motor areas of the cortex?
|
1. Primary motor area (M1) - precentral gyrus
• Homunculus! • Damage = UMN = contralateral flaccid paralysis → mild spasticity • Hemiparesis = distal limb muscles 2. Premotor cortex • Supplementary motor area, cingulate motor areas (medially) • Damage = apraxia (inability to performed learned movements w/o paralysis) • Damage to primary + premotor = full-blown UMN contralateral spastic hemiparesis 3. Frontal eye field - within premotor cortex • Damage = inability to look voluntarily to contralateral side • No paralysis 4. Broca's motor speech area - inferior frontal • Damage = expressive or motor aphasia |
|
|
What are the neuronal responses to injury:
1. Acute 2. Subacute and chronic 3. Axonal 4. Neuronal inclusions 5. Proteinopathies |
1. Acute - red neurons
2. Subacute and chronic - cell loss, reactive gliosis 3. Axonal - rounding, chromatolysis, margination of Nissl body (RER) to periphery 4. Neuronal inclusions - intranuclear viral inclusions, cytoplasmic accumulations (e.g. lipofuscin) 5. Proteinopathies - cytoplasmic aggregates or proteins with altered conformation (e.g. Alzheimer's neurofibrillary tangles, Parkinson's Lewy bodies) |
|
|
What are responses to injury in astrocytes?
|
1. Reactive gliosis
• Hypertrophy, hyperplasia • Gemistocytic astrocytes • GFAP stain 2. Alzheimer type II astrocytes • NOT Alzheimer's - metabolic disorders • Big pleomorphic nucleus (2-3x) 3. Rosenthal fibers • Cytoplasmic inclusions • Long-standing gliosis (e.g. slow tumors, Alexander disease), pylocytic astrocytoma 4. Corpora amylacea • Degenerative change • Aging, long-standing injury |
|
|
What are responses to injury in:
1. Oligodendrocytes 2. Ependymal 3. Microglia |
1. Oligodendrocytes
• Apoptosis - demyelinating disorders, leukodystrophy • Inclusions 2. Ependymal • Granulations - inflammation or hemorrhage • Inclusions - infections 3. Microglia • Proliferation - injury, infection → make <i>microglial nodules</i> around dying neurons = <i>neuronophagia</i> • Cytoplasmic accumulations - metabolic/storage disorders |
|
|
What are the CNS tissue reactions to injury?
|
1. Cerebral edema
• Vasogenic - extracellular • Cytotoxic - intracellular • Interstitial/hydrocephalic - ↑ CSF in obstructive hydrocephalus 2. Hydrocephalus • Disequilibrium between CSF production and resorption 3. ↑ intracranial pressure and herniation • Cingulate gyrus - anterior cerebral a. (subfalcine herniation) • Hippocampal gyrus - posterior cerebral a.; CN III, VI (uncal transtentorial herniation) • Upward herniation of mesencephalon and cerebellum through tentorial notch • Cerebellar tonsillar herniation through foramen magnum - ***LIFETHREATENING |
|
|
Which neurons have selective vulnerability to ischemia?
|
Vulnerable neuron cry "Peepeepee" → PPP
1. Pyramidal neurons in CA1 (hippocampus) 2. (Cerebellar) Purkinje neurons 3. Pyramidal neurons in neocortical layers III, V |
|
|
What are the gross findings with cerebral ischemia (acute, subacute, chronic)?
|
Acute/Subacute (48hrs): None
Subacute (2-10d): edema, pallor, obscured cortical gray/white matter junction Chronic (> 10d): liquefactive necrosis, cavitation |
|
|
What are the microscopic findings with cerebral ischemia (acute, subacute, resolving, remote)?
|
Acute (0-2d): red neurons
*Can persist for 2 weeks! Subacute: (2-10d): • PMNs - first 3d • Lipid-laden macrophages - > 3-5d Resolving (weeks to months): coagulative necrosis to liquefactive necrosis • Macrophages Remote (lifetime): glial-lined space with CSF, macrophages = "old cystic infarcts" |
|
|
Where are some common sites for lacunar infarcts? (4)
|
"BIT the Dust"
Basal ganglia Internal capsule Thalamus Deep white matter and pons |
|
|
What is CADASIL?
|
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
• Notch3 receptor gene mutation • Cause of recurrent strokes |
|
|
What is the most common cause of sub-arachnoid hemorrhage?
|
Saccular aneurysm → Circle of Willis
90% at arterial branch points in anterior circulation |
|
|
What is the pathology of vessels causing subarachnoid hemorrhage?
|
Loss of internal elastic lamina (IEL)
Muscular media replaced by hyalinized or fibrotic intima and residual adventitia |
|
|
What defines a transient ischemic attack (TIA)?
|
Stroke symptoms, but no infarct seen on MRI, CT
|
|
|
What are the different classifications/types of stroke? (5)
|
1. Atherthrombotic
2. Cardioembolic 3. Lacunar 4. Infrequent - arterial, blood, infectious, etc. 5. Cryptogenic = unknown |
|
|
What happens to cerebral autoregulation in ischemia?
|
Loss of ability to autoregulate because vessels are maximally dilated
→ changes in blood pressure can rapidly change cerebral blood flow |
|
|
What is the ischemic penumbra?
|
Area of brain that will infarct, but has not yet
Using MRI - perfusion weighted imaging (PWI) → can be a much larger area than part that is already infarcted |
|
|
What is the main goal of stroke therapy?
How is this achieved? |
Open the blocked artery ASAP - sooner = more penumbra saved = better outcomes
1. IV tPA - up to 4.5 hours from time of onset 2. Intra-arterial catheter-based methods - up to 6-8 hours |
|
|
When is reperfusion therapy indicated for stroke?
|
When there is a target mismatch (difference between DWI and PWI)
|
|
|
Where do most metastases to the brain arise?
|
Lung CA
Breast CA Melanoma |
|
|
What is the classic brain tumor headache?
|
Mild onset, usually in morning, goes away when get up
Gradually increases in frequency, duration, intensity May get worse on standing, Valsalva, ... |
|
|
What does GFAP stain for?
|
Cells of astrocyte origin
|
|
|
What is the most common malignant primary brain tumor?
|
Glioblastoma multiforme
|
|
|
What characteristics of tumors are more likely to cause seizures?
|
Low grade
Slow growing Superficial grey matter |
|
|
What mutation makes oligodendrogliomas more susceptible to chemo?
|
1p, 19q loss of heterozygosity
|
|
|
What is the cause of death for patients with oligodendrogliomas?
|
Transformation to anaplastic oligodendrogliomas
|
|
|
Why do meningiomas enhance so well with contrast?
|
They are outside the BBB
|
|
|
What might you see in someone with neurofibromatosis type 2?
|
Meningiomas
Bilateral acoustic neuromas (vestibular schwannomas) Ependymoma |
|
|
Why don't patients with acoustic neuromas present with vertigo?
|
Slow growing tumor, so vestibular system can compensate
|
|
|
What may be a confounding factor for increased prolactin that's NOT a prolactinoma?
|
Compression of pituitary stalk decreases dopamine (which normally inhibits prolactin)
|
|
|
What is pituitary apoplexy?
|
Spontaneous bleed/infarct of pituitary adenoma = sudden onset of neuro symptoms
Especially in pregnancy Medical emergency |
|
|
How does primary CNS lymphoma arise in the brain if there is no lymph tissue?
|
Nobody knows.
Hypotheses: 1. Tumors develop outside CNS and seed multiple organs → Immune system destroys the tumor outside the CNS 2. Lymphocytes get in after inflammation → become malignant |
|
|
What is the most common childhood CNS tumor? What is "school phobia"?
|
Medulloblastoma
Headache in the morning that goes away (brain tumor headache) → kids feel sick, don't go to school, then feel better |
|
|
What is one main symptom seen in craniopharyngioma NOT seen in pituitary adenoma?
|
Diabetes insipidus
|
|
|
What are the 4 categories of brain tumors?
|
Gliomas
Neuronal Poorly differentiated Meningiomas |
|
|
What are the criteria for grading glial tumors? (4)
|
Atypia (grade II)
Mitosis (grade III) Endothelial/vascular prolif (grade IV) Necrosis (grade IV) |
|
|
What are some TSG mutations in gliomas?
|
Low grade - p53
Primary glioblastoma - EGFR, PTEN Astrocytes - 10q/PTEN deletion |
|
|
What cancer is seen in the cauda equina?
|
Myxopapillary ependymoma
|
|
|
How is coma defined?
|
A state of unresponsiveness from which the patient cannot be aroused to respond appropriately.
|
|
|
What 3 things are required for full consciousness?
|
1. Ascending arousal system (reticular activating system)
• Pons, midbrain nuclei that project to (2) and (3) → herniation 2. Thalamus, hypothalamus (diencephalon) → basilar artery occlusion 3. Cerebral cortex → anoxic injury after cardiac arrest *Coma = damage to one of these systems (bilaterally, if thalamus or cortex) |
|
|
What are some types of herniation?
|
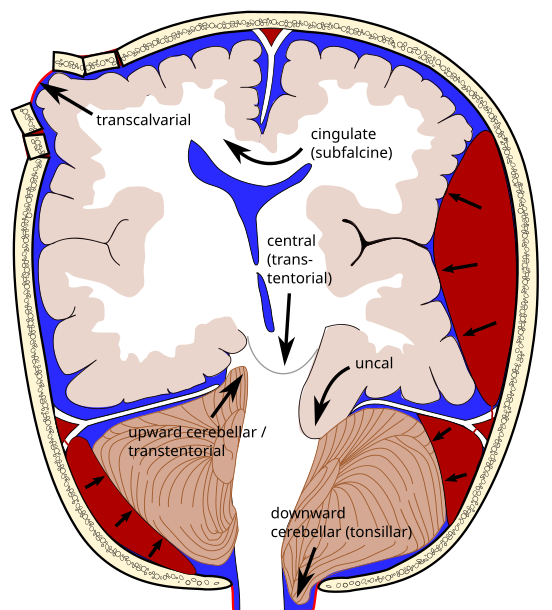
1. Uncal (transtentorial) temporal lobe shifts over free tentorial edge = compresses brainstem
2. Central - brainstem shifted downward 3. Subfalcine - cingulate gyrus shifts under falx cerebri (4. Transcalvarial) 5. Upward - posterior fossa protrudes up above tentorium = brainstem compression 6. Tonsillar - cerebellar tonsils protrude through foramen magnum = brainstem compression |
|
|
What are some common findings associated with uncal herniation?
|
1. Blown pupil
• Temporal lobe pushes up against CN III → has PSNS nerves 2. Hemiparesis (may vary which side, depending on what is compressed, so not very localizing) • Kernohan's notch 3. Occlusion of posterior cerebral artery |
|
|
How might oculomotor function help define a structural vs. metabolic cause of coma?
|
Asymmetric oculomotor function = structural (usually)
|
|
|
What kinds of pupils would you expect to see with the following lesions:
1. Diencephalic, metabolic 2. Pretectal 3. Midbrain herniation 4. Pontine |

1. Diencephalic, metabolic
• Small, reactive 2. Pretectal (e.g. upward herniation) • Pressure on pretectal nucleus = slightly enlarged, fixed to light 3. Midbrain herniation • CN III compression = unilateral dilated pupil ("blown") • Complete compression = damages sympathetics and parasympathetics = midposition, fixed pupils 4. Pontine (sympathetic) • Pinpoint pupils |
|
|
What is the Glasgow coma scale?
|
Used to describe a patient's state based on:
1. Eye opening 2. Best verbal response 3. Best motor response Scre of 3-8 = coma |
|
|
Describe:
1. Stupor 2. Vegetative state 3. Minimally conscious state 4. Akinetic mutism 5. Catatonia 6. Locked-in syndrome |

1. Stupor - reduced responsiveness requiring stimulation for arousal
2. Vegetative state - periodic wakefulness with total lack of cognition • Intact brainstem reflexes • No purposeful interaction 3. Minimally conscious state - severely impaired consciousness, but some evidence of awareness of self/environment 4. Akinetic mutism - silent, alert-appearing immobility • No volitional activity, "loss of motivation" → medial-frontal lobe damage 5. Catatonia - abnormal tone/movement, speech, activity • Normal brainstem reflexes, normal sleep-wake cycles 6. Locked-in syndrome - all 4 limbs + lower CNs paralyzed • Not disorder of consciousness, retained vertical eye movements • Pontine lesions |
|
|
What is brain death?
|
Irreversible cessation of all functions of the entire brain, including brainstem
• Cause must be irreversible structural or metabolic damage • Normal core temperature (not hypothermia) • Normal systolic temperature (not hypoperfusion) |
|
|
What are 2 really bad things that can happen to patients with traumatic brain injury?
|
Hypoxia (neuronal death)
Hypotension (↓ perfusion) |
|
|
What is the Monroe-Kellie doctrine?
|
Causes of ↑ intracranial pressure:
1. ↑ CSF 2. ↑ brain tissue (edema) 3. ↑ blood (hemorrhage) 4. Foreign body If one goes up, the others have to go down, or else there will be ↑ ICP |
|
|
What is the ischemia/ICP cycle?
|

↑ ICP = ↓ perfusion = ischemia = ↑ edema = ↑ ICP ...
|
|
|
What is the role of Ca in TBI?
|
Ischemia = ↑ Ca
= depolarization, unexcitability ↑ glutamate Ca-dependent ATPase activated = depletes energy stores → Cells unexcitable for 72 hours |
|
|
What is citicoline?
|
Antagonist to glutamate effects on ischemia and death
→ glutamate linked to neuronal excitation and death Not clear if useful for stroke or TBI |
|
|
What is the only neuroprotectant used clinically?
|
Hypothermia
Especially cardiac arrest Doesn't work in TBI |
|
|
What are some interventions for ↑ ICP?
|
Hypertonic saline
Mannitol Vasopressors (if ↓ cerebral perfusion) Drainage (hydrocephalus) |
|
|
Does giving a blood transfusion help with brain oxygen tension?
|
Yes, it increases brain oxygen tension.
Age of blood doesn't matter. Not clear if it's better than ICP monitoring therapy. |
|
|
What is diffuse axonal injury?
|
Acceleration/deceleration injury, rotational component
Microhemorrhages, white matter injury, especially corpus callosum MRI > CT |
|
|
What do steroids do in TBI? Albumin?
|
Both ↑ mortality...
(Steroids are good in some brain tumors) |
|
|
What happens to the alpha/delta ratio (EEG) in ischemia?
|
↓
|
|
|
What did the DECRA study show about decompressive craniectomy?
|
Decompressive craniectomy ↓ ICP more than standard care, but these patients had ↑ mortality
|
|
|
What is the function of:
Gs Gq Gi |
Gs
• Adenylate cyclase → ↑ cAMP → PKA Gq • PLC → Ca → PKC Gi • ↓ adenylate cyclase |
|
|
What are the MOAs of cocaine and amphetamine on monoamines?
|
Cocaine - inhibits monoamine reuptake
Amphetamine - increases monoamine release |
|
|
What are the four main dopaminergic pathways in the brain?
|
1. Mesolimbic
• Reward pathway • Addiction 2. Mesocortical 3. Nigrostriatal • Motor control • Degenerates in Parkinson's 4. Tuberoinfundibular |
|
|
Where do NE pathways reside in the brain? What is it's main function?
|
Locus ceruleus
Maintains attention, sets mood |
|
|
How do triptans treat migraines? (3)
What is a big potential adverse effect? |
1. 5-HT1B - constrict intracranial blood vessels
2. 5-HT1B, 1D, 1F - ↓ neuropeptide release (↓ inflammation) 3. ↓ nociceptive flow from vasculature to brainstem ***Also act on coronary vessels = ischemia risk |
|
|
Where do serotonin pathways reside in the brain? What does it do?
|
Raphe nucleus
Regulation of body temperature, sleep, mood, appetite, pain |
|
|
Where is histamine found in the brain? What does it do?
|
Hypothalamus
Sleep, attention, body temperature, pain |
|
|
What are the two main transmitters in the brain?
|
Glutamate (+)
GABA (-) |
|
|
How is the reuptake of glutamate different than other molecules?
|
Reuptake by glial cells
|
|
|
What is the function of:
AMPA NMDA Kainate Delta |
AMPA
• Fast excitatory transmission • Plasticity NMDA • Slow excitatory currents • Plasticity - memory and learning • Calcium signalling Kainate • Pre and post-synaptic • Modulatory Delta • Mystery • Purkinje cell development |
|
|
What is an interesting structural feature of NMDA receptors?
|
Require 2 agonists (glycine, glutamate) for signalling
• NR1 and NR2 subunits |
|
|
What is the different between neurotransmitters and neuropeptides?
|
Neuropeptides:
• Transcription of precursor mRNAs, alternate splicing • Packaging in Golgi • Use more prolonged stimuli, ↓ Ca |
|
|
What is the function of orexin (hypocretin)?
|
Maintenance of normal vigilance and muscle tone
|
|
|
What are the characteristics of Parkinsonism?
|
Tremor (resting)
Rigidity Akinesia Postural instability |
|
|
What is the pathogenesis of Parkinsonism?
|
Loss of SNc = ↑ D2 pathway (inhibitory), ↓ D1 (excitatory)
= bradykinesia |
|
|
What are some causes of Parkinsonism?
|
1. Idiopathic Parkinson's disease
2. Symptomatic parkinsonism • Drug-induced - neuroleptics (haloperidol), metoclopramide • Toxins - CO poisoning, Mn toxicity, MPTP • Metabolic • Vascular - microinfarcts • Post-encephalitic • Post-traumatic 3. Neurodegenerative |
|
|
What characterizes Parkinson's <i>disease</i>?
|
Tremor (resting) - not required
Rigidity Bradykinesia Postural instability - typically LATE **Response to levodopa!! |
|
|
What are some genes involved in Parkinson's?
|
α-synuclein
Parkin UCH-L1 Susceptibility genes **Also environmental factor |
|
|
What is the classic histopathological finding in Parkinson's?
|
Lewy bodies
(filled with α-synuclein) |
|
|
What are some nonmotor symptoms of Parkinson's?
|
Anxiety
Depression Sleep disturbance Constipation Olfactory deficit |
|
|
How is Parkinson's diagnosed?
|
Clinically, history
MRI - mostly to r/o other things SPECT - dopamine transporter uptake scans - look at presynaptic transporters • Diagnoses Parkinsonism - doesn't tell what kind |
|
|
What is progressive supranuclear palsy (PSP)?
|
Chronic, progressive, neurodegenerative > 40yo
Parkinsonism - • Symmetric, resting tremor uncommon • <b>Supranuclear gaze palsy</b> - doll's eye maneuver - can follow finger by moving head and eyes move, but can't send command to move them → vertical movements first, then horizontal • EARLY postural instability → Upright with head down (to get eyes to look up!) • EARLY dysphagia/dysarthria |
|
|
What is multiple system atrophy (MSA)?
|
MSA-P - parkinsonian
MSA-C - cerebellar Parkinsonian OR cerebellar features + autonomic dysfunction (orthostatic hypotension, urinary incontinance, erectile dysfunction) Cognitively intact Age 50s Antecollis - neck bent forward |
|
|
Describe the following characteristics of hyperkinetic disorders:
1. Tremor 2. Chorea 3. Dystonia 4. Tics 5. Myoclonus 6. Athetosis 7. Akathisia |
1. Tremor - involuntary rhythmic oscillatory
(• Resting - PD) • Action - postural (e.g. holding a book), kinetic • Essential tremor - most common 2. Chorea - irregular, unpredictable dancing movements - flow from one body part to another • STN not working 3. Dystonia - sustained but not fixed muscle contractions • Twisting, repetitive movements, abnormal postures • Agonist/antagonist muscles working at same time • <b>Geste antagoniste</b> - sensory trick that releases body part (e.g. to open eyes in blepharospasm, touch cheek) 4. Tics - intermittent, stereotyped movements or sounds **semi-voluntary • Motor • Vocal • Tourette's - motor + vocal, begin <21yo, wax and wane 5. Myoclonus - brief, lightning jerks • Asterixis - negative myoclonus (loss of tone) 6. Athetosis - continuous, slow writhing movement - usually more distal • Associated with chorea 7. Akathisia - subjective sensation of restlessness = can't keep still • Often neurolept |
|
|
What is Huntington's disease?
|
CAG repeat on chr.4
→ Loss of caudate Chorea Dementia Depression/psychosis (Juvenile bradykinetic/rigid form) |
|
|
Why is every kid who has dystonia given a trial of sinemet?
|
May be DRD = dopamine-responsive dystonia
Responds to L-DOPA, doesn't decrease responsiveness over time like PD, bad Dx to miss |
|
|
What is the drug of choice for treating tics?
|
Clonidine!
Most untreated - don't want to use psych drugs during development, and many go away on their own Also: • Counseling • Benzodiazepines • DA antagonists • Botox |
|
|
How is Parkinson's treated?
|
1. L-DOPA (dopamine precursor) + carbidopa (dopamine decarboxylase inhibitor)
• Dopamine can't get into CNS by itself • Dopa-decarboxylase in gut breaks down L-DOPA 2. MAO inhibitors (MAO degrades dopamine) • Selegiline - MAO-B inh. (less tyramine hypertensive crisis chance) 3. DA agonists • Bromocriptine • Pramipexole, Ropinirole 4. Anti-cholinergics • Benztropine • Trihexyphenidyl 5. COMT inhibitors + L-DOPA • Entacapone, Tolcapone 6. Amantadine • NMDA antagonist • DA reuptake inhibitor ?? |
|
|
Which Parkinson's treatments are disease modifying?
|
None...
|
|
|
What are some sites for deep brain stimulation surgery?
|
Thalamus
Globus Pallidus STN |
|
|
What is the difference in epidemiology between migraines and cluster headaches?
|
Migraines:
• Mostly women • Family history Cluster: • Mostly men • No family history |
|
|
What is the pathogenesis of migraine?
|
3 parts
1. Central - serotonergic transmission 2. Neurogenic - activation of trigeminovascular system and neuropeptide release 3. Vascular - perivascular (sterile) inflammation |
|
|
What causes visual aura?
|
↓ Mg in neurons of occipital cortex = NMDA receptors more sensitive to Glu, Asp
= occipital cortex hyperexcitable = photophobia • Wave of depolarization = scintillation • Wake of depolarization = area of brain that's relaxed ("spreading cortical depression") = scotoma *associated with spreading oligemia |
|
|
What is the role of the trigeminovascular system in migraine?
|
1. Spreading cortical depression impulses sent to trigeminal nucleus caudalis
2. Trigeminal axons activate trigeminovascular system = depletion of serotonin from nerve terminals 3. Release of neuropeptides • CGRP - vasodilation • Substance P, Neurokinin A - plasma protein extravasation = ↑ flow in dural arteries = pounding = transmitted to thalamus, cortex = PAIN |
|
|
What is a basilar migraine?
|
Aura/symptoms originate in posterior fossa
(wrongly attributed to basilar a.) Blindness (bilateral), vertigo/ataxia, nausea, emesis, bilateral parasthesia |
|
|
How are migraines treated?
|
Analgesic
Triptan Butalbitol DA agonists --- For status migrainosis: parenteral: Dihydroergotamine Steroids Divalproex (2 x valproate) |
|
|
What is used to prevent migraines?
|
Valproic acid (~35% reduction)
Topiramate (~40%) Propranolol (~60%) Botox Antidepressants (TCA, SSRIs) Verapamil |
|
|
Describe cluster headaches.
|
Cyclic, circadian severe unilateral periorbital pain
Rocks, paces in the dark Cranial autonomic activation 15-90 min |
|
|
Describe paroxysmal hemicrania.
|
Mostly women.
Similar to cluster. Sit quietly, hold head, go to bed. They respond completely to indomethacin. 5-45 min |
|
|
Describe SUNCT.
|
Sudden Unilateral Neuralgiform headache with Conjunctival injection and Tearing
Males 5 sec to 5 min long, up to 30 per hour |
|
|
What is a hypnic headache?
|
Women > 60
Hits a few hours after bed (1-3AM), lasts 15-60 min Responds to lithium, caffeine, melatonin, indomethacin (verapamil?, β-blockers?) |
|
|
What is a rebound headache?
|
Sensitization of pain pathways from overuse of analgesics,
>10d/mo for > 3mo More likely in migraneurs |
|
|
What are some examples of thunderclap headaches?
|
• Exertion headaches
• Reversible cerebral vasoconstriction (Call-Fleming) • Hemorrhage, aneurysm, dissection, ... • Chiari I • Colloids |
|
|
What are weak and strong opioids?
|
Weak - low mu affinity and/or not in Schedule I/II
• Codeine, hydrocodone, buprenorphine, tramadol Strong - everything else (**Excluded - antitussives, antidiarrheals) |
|
|
What are the 4 classes of opioids?
|
1. Agonists (μ)
• Morphine, meperidine, hydromorphone, ... 2. Antagonists (μ) • Naloxone, naltrexone 3. Mixed agonists (κ)/antagonists (μ) • Pentazocine, butorphanol, nalbuphine 4. Partial agonists (μ) • Buprenorphine - very high affinity but low effect = if someone does another drug, it has very little effect |
|
|
What are the 3 opioid receptors?
|
Mu, Kappa, Delta
• One gene for each • Subtypes from splice variants Mu: • Analgesia, "euphoria", dependance, <b>respiratory depression</b>, miosis, GI effects, pruritis Kappa: • Mild analgesia • Less respiratory depression • Psychomimetic effects Delta: • Unknown |
|
|
How are opioids given clinically?
|
Pick an opioid
Titrate it until: a) Patient feel better or b) Side effects w/o much analgesia → Change the opioid!! • Opioids have different receptor specificities • Individuals have different receptor densities |
|
|
What descending pathway do opioids activate?
|
Periaqueductal Gray
• Releases endorphins |
|
|
What are the PK of opioids?
|
Most - 2-3hr elimination half-life
Methadone - 24hr! (average) |
|
|
What are the PD of opiods?
|
1. Analgesia
2. Respiratory depression 3. Euphoria/dysphoria 4. Nausea/vomiting (via area postrema) 5. Constipation (↓ peristalsis) (6. ↑ sphincter tone) |
|
|
What is opioid miosis?
|
Pupillary constriction
• Seen with all opioid agonists • Dose-dependent • NO TOLERANCE to miosis • Mediated by parasympathetics (reversed by atropine) |
|
|
What are the symptoms of opioid withdrawal?
|
Sympathetic overdrive:
• Sweating, HTN, tachycardia • Mydriasis • Hyperventilation • Diarrhea, abdominal cramping **NON-LETHAL!! (unlike benzos, ...) |
|
|
What is the concern with sustained release formulations?
|
If chewed or crushed, full dose at once!
|
|
|
How is pain classified "practically"?
|
1. Acute
• Goal: Treat until you can fix underlying cause 2. Chronic • Serves no biologic function anymore • Persists despite treatment of known cause • Goal: maximize function independent of health care system |
|
|
What are some ways pain is modulated?
|
1. Aβ fibers - decrease firing (touch when hurt yourself)
2. Descending pathways (Limbic system to Periaqueductal gray) - context of pain can make it better/worse |
|
|
What is allodynia?
|
Pain with touch
|
|
|
What drives peripheral sensitization?
|
PGE2!
Sensitize nerve endings, make area painful to touch after injury (e.g. sunburn) |
|
|
What is central sensitization? What are its mechanisms? (3)
|
Ramps up pain expression so you don't mess with a wound
1. Wind up • Triggered by NMDA receptor • ↑ pain sensation from ↓ activation of C-fibers 2. Neural sprouting • Aβ fibers sprout to become positively triggering pain, bypassing opioid receptors 3. CNS prostaglandins • ↑ Substance P • ↑ firing from secondary neurons • ↓ inhibitory input from brain = ↑ PAIN transmission **NSAIDs work without inflammation! = opioid resistant pain |
|
|
What is the placebo effect?
|
Improvement in symptoms with "fake" treatment
• Naloxone-reversible!! = descending pathways involved! |
|
|
What medications are used for chronic pain other than opioids?
|
1. Antidepressants
• TCAs, SNRIs → block reuptake of 5-HT, NE (block pain transmission) • Enhance descending inhibitory system • NOT by treating depression • 2-4 weeks to see effect • Mild/moderate • NO tolerance!! 2. Anti-epileptics - Gabapentin, Pregabalin • Lancinating ('lightening') neuropathic pain 3. Muscle relaxants • Mechanism similar to alcohol • TOLERANCE • Carisoprodol → metabolized to meprobamate, an addictive tranquilizer with severe withdrawal |
|
|
How do Schwann cells differ from oligodendrocytes?
|
Both provide myelin
Schwann cells myelinate ONLY ONE NERVE |
|
|
What is compact/non-compact myelin?
|
Compact - main function of myelin for nerve conduction
• Protein zero (P0) - compaction • PMP22 - myelin regulation Non-compact - allows diffusion of nutrients/ions through myelin, axon • Connexin 32 (Cx 32) - communication between myelin loops |
|
|
What is dystrophin?
|
Sarcolemmal protein that connects actin cytoskeleton to extracellular matrix
*Necessary to transfer force of myofibril contraction to body Duchenne, Becker muscular dystrophy |
|
|
What are type I and type II muscle fibers?
|
Type I
• Slow, small, ↓ fatigue • ↑ mitochondria, ↑ myoglobin = oxidative metabolism Type II • Large, fast, ↑ fatigue • ↑ SR, ↑ glycolytic enzymes = anaerobic metabolism |
|
|
What are the main types of axon injury?
|
1. Wallerian degeneration
• Focal damage to axon → distal portion degenerates • Trauma, ischemia, underlying abnormality 2. Primary neuronal degeneration (neuronopathy) • Death of cell body → degeneration of nerve • Anterior horn cell (motor), dorsal root ganglion (sensory) 3. Distal axon degeneration (dying back neuropathy) • Degeneration of the distal nerve that degenerates more proximally over time • Death from lack of nutrients, ↓ axoplasmic flow |
|
|
What are the types of myelin injury?
|
1. Segmental
• Usually acquired 2. Uniformly • Usually hereditary (all of myelin affected) |
|
|
When might you see onion bulbs?
|
Chronic demyelinating disease, usually hereditary
Caused by constant damage and remyelination to axons |
|
|
What are the 2 ways axons can restore connection (e.g. Wallerian degeneration)?
|
1. Regrowth guided by Schwann cells (Bands of Bunger)
2. Collateral sprouting from neighboring motor units *May change muscle fiber type = fiber type grouping (not random checkerboard anymore) |
|
|
What are some big differences between neuropathy and myopathy?
|
Myopathy
• No sensory loss • Proximal symmetric muscle weakness Neuropathy: usually length dependent (distal > proximal) |
|
|
What are some characteristics of Guillain-Barre syndrome? (sp. Acute inflammatory demyelinating polyradiculopathy - AIDP)
|
Myelin problem = Large fibers
• Post-infectious (GI, respiratory often) = immune-mediated segmental demyelination from molecular mimicry • Evolves over days up to 4 weeks • Proximal and distal motor/sensory deficits, absent reflexes **Autonomic instability and respiratory failure |
|
|
How is AIDP diagnosed? Treated?
|
• ↑ CSF protein with no mononuclear cells in CSF
• Slowed conduction on EMG Tx: • Plasma exchange or IVIg • NOT NOT NOT steroids • Supportive (cardiac, respiratory, ...) |
|
|
What is CIDP?
|
"Chronic" version of AIDP
Disease continues to progress after many weeks Steroids very important |
|
|
What is Charcot-Marie-Tooth? What are the types? How does it present?
|
Heriditary demyelinating neuropathy
CMT 1 - demyelinating CMT 2 - axonal CMT X - demyelinating • < 20s • "Stork leg" appearance • Foot weakness, motor (and sensory) loss, deformities • ↓ conduction velocity, onion bulbs |
|
|
What are some types of CMT? (3)
|
CMT1A
• AD • Most common • Duplication of PMP22 (compact myelin) → maintains structural integrity, apoptosis, regulation, ... CMT1B • AD • Mutation of MPZ (P0) - compact myelin → maintains tight myelin compaction CMT X • X-linked dominant • Mutation of Connexin 32 (non-compact) → Gap junction protein, involved in diffusion of ions, nutrients |
|
|
What are characteristics of axonal neuropathies?
|
• Length dependent (distal to proximal)
• Patchiness following individual nerves (vasculitis, compression) |
|
|
What is the most common cause of peripheral neuropathy?
|
Diabetes!
|
|
|
What do conduction studies in diabetic neuropathy show?
|
Axon loss
= ↓ amplitude = normal speed Affects feet more than hands |
|
|
What vitamin can cause axonal neuropathy?
|
B12 deficiency
Numbness, sensory ataxia, CST May have UMN findings and LMN (e.g. no ankle reflexes but brisk other ones) |
|
|
How many axonal neuropathies are idiopathic?
|
~1/3
|
|
|
What are some characteristics of vasculitic neuropathy?
|
NOT distal → proximal, but patchy and follow individual nerve
Rapid, painful onset - sensory + motor in named nerve distributions |
|
|
What is an example of a hereditary axonal neuropathies?
|
CMT 2
• Usually by 20s, 30s • Distal weakness • Stork legs • Atrophy ↓ sensory, motor amplitude |
|
|
What are the 3 main types of inflammatory myopathies?
|
1. Dermatomyositis
• Muscle + skin • Proximal weakness • Responsive to immunomodulating 2. Polymyositis • Muscle + other tissues • Proximal weakness • Responsive to immunomodulating therapy 3. Inclusion body myositis • Muscle • Patterned (finger/wrist flexors, quads) • NOT responsive to immunomodulating therapies |
|
|
What are some differences between dermatomyositis and polymyositis?
|
DM: any age | PM: > 20
DM: rash | PM: no rash DM: humoral | PM: cell-mediated DM: perifascicular atrophy Both get cardiac, pulmonary problems |
|
|
What is inclusion body myositis?
|
• Age > 50
• Finger/wrist flexors, Quads, dysphagia • VACUOLES!! |
|
|
What is muscular dystrophy?
|
Hereditary, progressive
Caused by mutations in proteins Usually proximal muscles affected first |
|
|
What are Duchenne and Becker muscular dystrophy? What is the main difference?
What are some signs/symptoms of them? |
Duchenne = wheelchair bound by age 12 (Becker, later)
Duchenne treated with prednisone, Becker isn't. • X-linked recessive • Calf pseudo-hypertrophy • Proximal weakness • Gower sign (getting up from the floor) |
|
|
What is myotonic dystrophy? What is a very common comorbidity?
|
Present with myotonia - delayed relaxation following contraction
DM1 - channelopathy • CTG expansion of myotonin protein kinase gene = messes up proteins, including Cl channels • <b>Cardiac arrhythmias in 90%!!</b> • Weak facial muscles - droopy, "hatchet" face DM2 - CCTG expansion on zinc finger protein 9 Severity correlates with size of expansion |
|
|
What are the 2 types of metabolic muscle disorders?
|
Glycogen
Lipid |
|
|
What is Pompe disease?
|
• AR
• Acid α-glucosidase deficiency = Accumulation of glycogen in lysosomes and cytoplasm • Infantile - weak and floppy from birth + early death • Adult - 30s/40s, proximal weakness Tx: IV α-glucosidase replacement |
|
|
What is McArdle disease?
|
• AR
• Myophosphorylase deficiency = can't mobilize glycogen (↓ breakdown) • Cramps, exercise intolerance + second wind phenomenon Tx: oral sucrose before exercise, slow warm up period |
|
|
What are disorders of lipid metabolism?
|
Trouble with prolonged activity of muscles
Carnitine deficiency: • Impaired transport of FFAs into mitochondria • Progressive weakness, difficulty with sustained activity • Oral carnitine supplement |
|
|
What are some stains that help diagnose metabolic muscle disorders?
|
1. PAS - glycogen
2. Oil O - lipid 3. Gomori trichrome - mitochondria |
|
|
What is steroid myopathy?
|
↑ glucocorticoids (often iatrogenic)
• ↓ protein synthesis, ↑ degradation, impaired mitochondrial function • Proximal weakness *Mostly affects type 2 fibers |
|
|
What is a common drug that causes myopathy?
|
Statins
|
|
|
What is a post-synaptic disease of the NMJ? Pre-synaptic diseases?
|
Post: Myasthenia gravis
Pre: LEMS, Botulism |
|
|
What are two diseases that commonly look like ALS?
|
Cervical spondylosis
• Natural wear-and-tear of age Kennedy's disease • CAG repeat • Androgen receptor involvement |
|
|
What is the most common cause of familial ALS?
|
SOD mutation "gain of function"
• Oligomerization (aggregation) • Oxidative damage |
|
|
What mutations are seen in sporadic cases?
|
FUS, TDP
MUCH more common than familial |
|
|
What is the glutamate hypothesis in ALS?
|
↑ glutamate released from pre-synaptic
→ NMDA → non-NMDA = ↑ Ca to post-synaptic neuron = mitochondrial damage |
|
|
What is the only FDA-approved treatment for ALS?
|
Riluzole
↓ glutamate release Only 2-3 mo increase in life, little improvement in symptoms |
|
|
What is the best thing you can do for your ALS patient?
|
Send them to specialty multidisciplinary ALS center
• Physical therapy, occupational therapy • Respiratory treatment etc. |
|
|
What is multiple sclerosis?
|
Genetic + environmental factors = autoimmune destruction of myelin
*May involve gray matter *May involve axonal disease Defined as lesions of the CNS with dissemination in time and space |
|
|
What is an exacerbation of MS?
|
Neurological disturbance lasting at least 24 hours (w/o fever or infection)
|
|
|
How does MS present?
|
1. Sensory disturbance (numbness, tingling)
2. Motor disturbance 3. Optic neuritis • Monocular vision loss • Impaired color vision • Pain with eye movement • Centrocecal scotoma 4. Brainstem/cerebellar • Vertigo • Diplopia |
|
|
Describe the paroxysmal symptoms of MS:
1. Lhermitte's 2. Uhtoff's phenomenon 3. Trigeminal neuralgia 4. Tonic spasms |
1. Lhermitte's
• Flex head forward → brief electrical shock down spine 2. Uhtoff's phenomenon • Recurrence of neurological symptoms with elevated body temperature (illness, heat, exercise, etc.) 3. Trigeminal neuralgia • Brief, shock-like facial pain - CN V root entry lesion 4. Tonic spasms • Brief stereotypical tonic contractions - seconds to minutes |
|
|
What are some findings on exam in MS?
|
1. Visual findings
• Afferent pupillary defect (doesn't constrict with light) • Optic pallor - pale disc, over time • Nystagmus • Internuclear ophthalmoplegia - medial longitudinal fasciculus (MLF) - adducts/abducts eyes together 2. Sensory 3. UMN deficits |
|
|
What are Dawson's fingers?
|
Typical MS lesions that occur perpendicular to the ventricles in sagittal cut
|
|
|
What do dark spots on T1-weighted imaging tell you?
|
Axonal degeneration
|
|
|
How is MS diagnosed?
|
Lumbar puncture - oligoclonal IgG bands
MRI - plaques (white matter demyelination) |
|
|
What are the criteria for establishing dissemination in space (DIS) and time (DIT)?
|
DIS: at least 1 T2 lesion in 2/4 areas of the CNS
• Periventricular • Juxtacortical • Infratentorial • Spinal cord DIT: 1. New T2 and/or Gd-enhancing lesion on follow-up MRI OR 2. Simultaneous presence of asymptomatic Gd-enhancing and non-enhancing lesions at one time. |
|
|
What % of MS patients present with the relapsing-remitting form?
Secondary progressive MS? Primary progressive MS? |
85%
50% of those 10% - no relapses but progressive (Can also have progressive relapsing - rare!) |
|
|
How are acute MS exacerbations treated?
|
IV methylprednisolone (aka IV salu-medrol = IVSM)
Rarely, plasma exchange |
|
|
What are the preventative treatments for MS?
|
1. Interferon
• IFN-β1a • IFN-β1b *↓ MMPs, → chemokine receptors, ↓ T-cell influx into CNs 2. Glatiramer acetate • ↓ TH2 cell traffic at BBB 3. Mitoxantrone • Chemotherapeutic agent **Cardiac toxicity 4. Natalizumab • Blocks T-cell interaction with VCAM-1 = can't enter CNS **Risk of Progressive Multifocal Leukoencephalopathy (PML) 5. Fingolimod • ↓regulates S1P on lymphocytes = prevents egress from lymphoid tissues = ↓ infiltration to CNS **None of these treat primary progressive MS! |
|
|
Neutralizing antibodies can mess up treatment with IFN-β and natalizumab.
|
True
|
|
|
What is dalfampridine?
|
K-channel blocker
Restores conduction of action potentials *Walking pill* - improves gait! |
|
|
What is dextromethorphan/quinidine used to treat in MS?
|
Pseudobulbar affect - involuntary laughter/crying that is incongruous/disproportional to emotional state
|
|
|
What is neuromyelitis optica (Devic's disease)?
|
Classically:
<b>1. Bilateral optic neuritis 2. Acute myelitis 3. 2/3: • Long spinal cord lesions (unlike discrete lesions in MS) • Brain MRI not typical for MS • NMO Ab (anti-Aquaporin 4) </b> **Often associated with autoimmune diseases (Sjogrens, Lupus) Tx with: IV methylprednisolone and plasma exchange *Limited data... |
|
|
What is Acute Disseminated Encephalomyelitis (ADEM)?
|
*Post-infectious or post-vaccinal encephalomyelitis
• Demyelinating • Children > adults Mental status changes, seizures Numerous, large enhancing MRI lesions Tx: IV methylprednisolone, plasma exchange 1/3 pts will go on to develop MS |
|
|
What is the function of semicircular canals? Otoliths?
|
Semicircular canals = angular rate sensors
• Horizontal • Anterior • Posterior Otoliths = linear accelerometers • Utriculus - horizontal • Sacculus - vertical (sagittal) **Also register tilt |
|
|
What is the vestibuloocular reflex (VOR)? Vestibulospinal reflex (VSR)?
|
VOR - allows us to see while head is moving (eyes focused)
VSR - balance |
|
|
What are the results of imbalance in VOR:
1. Both sides 2. One side 3. One horizontal canal 4. One vertical canal 5. Central |
1. Both sides - no nystagmus
2. One side - lateral, rotatory sensations 3. One horizontal canal - lateral nystagmus 4. One vertical canal - mixed vertical, rotatory 5. Central - vertical or horizontal eye jumping |
|
|
What is benign paroxysmal positional vertigo (BPPV)?
|
20% of all dizziness
+ Dix-Hallpike - stimulation of posterior semicircular canal • Eyes go upwards and twist into plane of canal Diagnostic of BPPV! Pathology: particles of limestone in utricle Tx: Epley maneuver - turn the head in a series of positions to roll rocks back into central part |
|
|
What is vestibular neuritis?
|
Vertigo, nausea for about 2 weeks
Thought to be viral infection (HSV) Superior canal, lateral canal, utricle *Posterior canal spared - but bad if they get BPPV! Tx: • Steroids • Vestibular suppression (Meclizine, phenergan, BZDs) |
|
|
What is bilateral vestibular loss?
|
Gentamycin!!!
→ no hearing loss, just dizziness Oscillopsia - loss of VOR = eyes move with head Ataxia - Romberg + Damage to hair cells → often permanent **Don't give vestibular suppressants |
|
|
What is Meniere's disease?
|
Dilation and episodic rupture of inner ear membranes
• Episodic vertigo • Tinnitus • Fluctuating hearing Most resolve within 2d, but return every 3mo Tx: • Vestibular suppressants • Antiemetics • Low-dose intratympanic gentamicin *Prevention: low salt diet, diuretics |
|
|
What is migraine associated vertigo?
|
Mostly middle-aged women
Headaches + dizziness with no other explanation Respond to triptan or prophylaxis (topiramate, propranolol, botox, verapamil,...) |
|
|
What are leukodystrophies?
|
Myelin abnormalities
Inherited enzyme mutations |
|
|
What is Krabbe disease?
|
• AR
• ↓ galactocerebroside β-galactosidase * Galactocerebroside accumulates in macrophages Sx around 3-6mo → die around 2 |
|
|
How is Krabbe disease diagnosed?
|
↓ galactocerebroside
MRI: diffuse, symmetric white matter involvement ↑ CSF protein ↓ nerve conduction velocities |
|
|
How is Krabbe disease treated?
|
Pre-symptomatic: stem cell transplant
Symptomatic = no treatment |
|
|
What is metachromatic leukodystrophy?
|
• AR
• ↓ arylsulfatase A enzyme • Infantile, juvenile, adult *Myelin accumulates in lysosomes Sx around 2-4yo → gait abnormalities, weakness, ... |
|
|
How is metachromatic leukodystrophy diagnosed?
|
↓ arylsulfatase A
MRI: subcortical demyelination - white matter abnormalities often <i>posteriorly</i> • Sparing of U fibers ↑ CSF protein ↓ nerve conduction velocities |
|
|
How is metachromatic leukodystrophy treated?
|
Supportive only...
|
|
|
What is adrenoleukodystrophy?
|
• XLR
• Inability to catalyze very long chain fatty acids (VLCFA) = loss of myelin, gliosis, lymphocytic infiltration 1. Childhood cerebral form 2. Adrenomyeloneuropathy *Accumulation of FAs damages adrenals, white matter → <b>adrenal insufficiency</b> Sx between 5-10yo, often boys → change in behavior, gait/coordination problems Often misdiagnosed with ADHD |
|
|
How is adrenoleukodystrophy diagnosed?
|
↑ plasma VLCFAs
Adrenal insufficiency MRI: T2 hyperintensity in white matter, posterior predominance too |
|
|
How is adrenoleukodystrophy treated?
|
Steroid replacement
Early symptomatic stem cell transplant |
|
|
What is Pelizaeus-Merzbacher disease?
|
• XLR
• Defective synthesis of myelin sheath protein → Proteolipid protein (PLP) gene Sx infancy/early childhood → nystagmus, choreoathetosis, hypotonia *Death by 5-7yo |
|
|
How is Pelizaeus-Merzbacher diagnosed?
|
PLP-1 mutation
MRI: diffuse hypomyelination Normal CSF protein, normal conduction velocity Autopsy - tigroid appearance of white matter (patchy!) |
|
|
How is Pelizaeus-Merzbacher treated?
|
Symptomatic
|
|
|
What is Canavan disease?
|
• AR
• ↓ aspartoacylase Sx <6mo → psychomotor arrest, <b>macrocephaly</b>, blindness |
|
|
How is Canavan disease diagnosed?
|
↑ N-acetylaspartic acid
MRI: diffuse ↑ lucency of white matter Normal CSF protein, normal conduction velocity |
|
|
How is Canavan disease treated?
|
Symptomatic
|
|
|
What is Alexander disease?
|
• GFAP protein mutations
= Rosenthal fibers Sx < early childhood → megacephaly, psychomotor regression, seizures |
|
|
How is Alexander disease diagnosed?
|
MRI: leukodystrophy with frontal predominance
NO optic atrophy (unlike Canavan) |
|
|
What is vanishing white matter disease?
|
• AR
• Loss of eIF2B function (many mutations) = important for protein synthesis and regulation under stress Sx: normally develop with some cerebellar ataxia <b>Acute worsening with illness, fever, trauma, fright</b> |
|
|
How is vanishing white matter disease diagnosed?
|
MRI: progressive replacement of white matter by CSF → strands!
|
|
|
How is vanishing white matter disease treated?
|
Avoid triggers
|
|
|
What are the two main differences between benzodiazepines and barbiturates in terms of MOA?
|
Benzodiazepines: increase frequency of conduction of GABA<sub>A</sub>, do not agonize it itself
Barbiturates: increase DURATION of GABA<sub>A</sub> opening, can agonize it at higher doses |
|
|
What drives the short-acting nature of barbiturates?
|
Redistribution to the tissues
|
|
|
Why can't you use thiopental in a continuous infusion to maintain anesthesia?
|
Half-life is dependent upon infusion time.
= it builds up to toxic levels in tissues |
|
|
What is flumazenil?
|
Antagonist of the benzodiazepine binding site on GABA<sub>A</sub>
= reverse effects from overdose or accelerate recovery |
|
|
What is the only IV anesthetic that is anti-emetic? Analgesic?
|
Propofol
Ketamine |
|
|
How is potency of inhaled anesthetics assessed?
|
Minimum alveolar concentration
Lipid solubility |
|
|
What is the most metabolized inhaled anesthetic?
Least? |
Halothane
Desflurane Nitrous oxide |
|
|
When are inhaled anesthetics absolutely contraindicated?
|
Malignant hyperthermia
Succinylcholine |
|
|
What are the main variables that determine PK of volatile anesthetic agents? (5)
|
1. Blood/gas partition coefficient
• ↑ solubility in blood = slower time to equilibrium 2. Blood flow • ↑ flow to tissue = ↑ uptake 3. [ ] in inspired air 4. Pulmonary ventilation 5. Pulmonary blood flow • ↑ flow = ↓ rate of rise of arterial tension Why?? • ↑ flow = ↑ blood exposed to anesthetic = ↑ "capacity" • ↑ anesthetic delivered to moderate/slow equilibrating tissue (because of ↑ flow) |
|
|
What type of stroke requires early surgical intervention?
|
Cerebellar hemorrhage
|
|
|
What are the main causes of hemorrhagic stroke (intracerebral)? (5)
|
1. HTN
• Seen in pts 50-70yo 2. Cerebral amyloid angiopathy • Disease of aging - > 70yo • Associated with Alzheimer's • <b>NEVER give anticoagulation, even if a-fib</b> 3. AVMs, cavernous malformations, AV fistulas 4. Drugs • Cocaine, amphetamine • Due to rupture from high BP (first-time user) OR due to vasculitis (long-time user) 5. Iatrogenic • Anticoagulants, antiplatelets *Any pt on blood thinner with new neuro symptoms = stroke unless proven otherwise |
|
|
What is clot expansion?
|
Expansion of the bleed
• Acute - within 15-20 mins • 1/3 of pts have it within 24 hours |
|
|
How is intracerebral hemorrhage detected?
|
Acute: MRI = CT
Subacute/chronic: MRI >> CT |
|
|
How is intracerebral hemorrhage treated?
|
1. ↓ ICP
2. Reverse coagulopathy 3. Surgery • Cerebellar bleed 4. Supportive care, rehab |
|
|
What are the risk factors for subarachnoid hemorrhage?
|
Female
HTN Smoking |
|
|
How is a subarachnoid hemorrhage described when it presents?
|
Sudden headache, worst of your life
Must be evaluated immediately |
|
|
How is subarachnoid hemorrhage evaluated and treated?
|
CT, MR (with angiography), LP if needed
Tx: Surgery Endovascular coiling Prevent complications (rebleed, vasospasm, hydrocephalus, seizures, SIADH) |
|
|
What structure is responsible for inducing ectoderm to become future neural tube?
|
Notochord
|
|
|
What are the defects of primary neurulation?
|

1. Diastatomyelia
• Notochord splits around adhesion between endoderm/ectoderm **Split notochord - most severe form = connection between intestinal cavity (endoderm) and dorsal skin in midline = enterric fistula 2. Spinal lipomas (spina bifida occulta) • Premature separation of neural ectoderm from cutaneous ectoderm • Mesenchyme enters ependyma of neural tube inducing fat formation 3. Dermal sinus and Myelomeningocele • Incomplete separation of neuroectoderm from cutaneous ectoderm • Focal = dermal sinus • Diffuse = myelomeningocele |
|
|
What is tethered cord syndrome?
|
Problem with secondary neurulation
Fatty filum, low-lying conus General symptoms Arnold-Chiari, Hydrocephalus |
|
|
When should women of child-bearing age take folate?
|
ALL women of child-bearing age should take folate, because neural tube forms before most pregnancies are known.
|
|
|
Early brain cells = ____
Late brain cells = ____ |
Early = deep
Late = superficial "Inside out" |
|
|
What are the two facets of neuroblast migration and what chemicals control them?
|
1. Radial migration directed by glia
• Glutamatergic 2. Tangential migration from lateral ganglionic eminence (LGE) to MGE directed by GABA |
|
|
What is tuberous sclerosis?
|
Abnormal proliferation of neuron and glia
→ Giant cell astrocytoma |
|
|
What is hemimegalencephay?
Microlissencephaly? |
Too much proliferation!!
Too little proliferation... |
|
|
What is focal cortical dysplasia?
|
Seizures!
Gyral irregularities Can be with signal change or without (MRI) |
|
|
What is lissencephaly?
|
Smooth brain = no gyri/sucli
LIS1 (P>A), DCX genes (A>P) - 80% of cases • <b>Microtubule associated</b> DCX = males Cobblestone - type II • Muscle eye brain • Walker Warburg • Fukuyama • Merosin negative congenital muscular dystrophy |
|
|
What is subcortical band heterotopia?
|
Bands of grey matter interposed between cortex and lateral ventricle.
LIS1, DCX types (same genes) DCX = females *Also periventricular type |
|
|
What is polymicrogyria?
|
Very variable
Male > female Bilateral, perisylvian regions Bilateral generalized Bilateral frontal Schizencephaly - cleft in continuity from left ventricle to subarachnoid space lined by polymicrogyri |
|
|
What do malformations of cortical development typically cause?
|
1. Epilepsy
2. Intellectual disability 3. Congenital neurological deficity 4. Cerebral palsy |
|
|
What are some areas of the brain involved in sleep?
|
Brainstem
Hypothalamus SCN Thalamus |
|
|
What are some modulators of wakefulness?
|
ACh
DA Histamine 5-HT NE Orexins (+ feedback to all of these) |
|
|
What controls NREM sleep?
|
GABA secreted from the ventrolateral preoptic area (hypothalamus)
|
|
|
What controls REM sleep?
|
<b>↓ DA, NE</b>
= ↑ ↑ ACh to cortex = activates mind Stimulates glycine in cortex = muscle atonia |
|
|
What is the role of the SCN?
|
With light, causes wakefulness.
Also activates sympathetic nerves to pineal gland, results in melatonin release that has negative feedback on SCN = causes sleep eventually |
|
|
What is restless leg syndrome? (4)
|
1. Urge to move legs, associated with pressure or discomfort
2. Worse at times of rest (e.g. opera) 3. Relieved with movement 4. Worse at night |
|
|
What is the pathophysiology of restless leg syndrome?
|
1. DA
• Improves with L-DOPA, pramipexole • ↓ D2 activity? 2. Iron • ↑ RLS with Fe deficiency • ↓ CSF Ferritin • MRI - ↓ Fe in basal ganglia • Fe is a cofactor in tyrosine hydroxylase - role in D2 receptor |
|
|
What is the treatment for circadian rhythm disorders?
|
Advanced phase - light therapy in the evening
Delayed phase - light therapy in the morning, melatonin at night |
|
|
What is the issue with primary insomnia?
|
NO underlying medical/psych disorder
Usually with life stressor (e.g. baby), but it persists after the stressor goes away because it becomes a learned behavior |
|
|
How is primary insomnia treated?
|
• CBT
• BZDs, zolpidem, sedating antidepressants • OTC medications |
|
|
How does Ramelteon work to cause sleep?
|
Agonist of the SCN via MT1/MT2 (melatonin) receptors
|
|
|
What is narcolepsy?
|
↓ hypocretin and hypocretin neurons
Excessive daytime sleepiness REM-like phenomenon during wakefulness • Cataplexy with emotions • Hypnagogic hallucinations • Sleep paralysis (↓ orexin = ↓ catecholamines = paralysis from ACh) |
|
|
What is idiopathic hypersomnia?
|
Unlike narcolepsy:
• Deep sleep • Unrefreshing naps • No REM phenomena |
|
|
How is narcolepsy treated?
|
↑ DA release (DA promotes wakefulness)
Amphetamines → CV side effects Modafinil → better profile, ↓ DA reuptake, ↓ NE reuptake Sodium Oxybate - endogenous GABA metabolite • Acts on GABA<sub>B</sub> and GHB • ↑ slow-wave sleep, but not clear how it improves daytime alertness |
|
|
What is parasomnia?
|
Unpleasant or undesirable behaviors during sleep - REM or NREM
• State dissociation between awake, REM, NREM 1. Sleepwalking (somnambulism) - NREM • Disorder of arousal • Children > adults • Correct exacerbating cause (e.g. apnea), clonazepam 2. Night terrors - NREM • Children >> adults • Often precipitating factor 3. REM sleep behavior disorder • Complex dreams with motion (disruption of atonia) • ↑ in α-synucleopathy (PD, MSA) • Clonazepam |
|
|
What does it mean if a patient has bilateral motor manifestations (e.g. clonus) affected but no loss of consciousness?
|
Both hemispheres involved → must lose consciousness
Not a seizure - usually One exception: supplementary sensory motor area - extreme agitation and movement, rarely recognized as epileptic |
|
|
What are the targets for anti-convulsant therapy?
|
1. ↑ GABA
2. ↓ Glutamate 3. Block inward Na+, Ca2+ currents 4. ↑ outward K+ currents |
|
|
What are some P450 inducers within AEDs? Inhibitors?
|
Carbamazepine
Phenytoin Phenobarbitol Valproate |
|
|
What are two examples of drugs that can exacerbate epileptic seizures?
|
Tramadol
Venlafaxine |
|
|
What are some AEDs used for:
1. Neuropathic pain 2. Bipolar disorder 3. Migraine |
1. Neuropathic pain
• Gabapentin • Pregabalin • Carbamazepine 2. Bipolar disorder • Lamotrigine • Valproate 3. Migraine • Valproate • Topiramate • Zonisamide |
|
|
What are the 3 ways to get CNS infection?
|
1. Hematogenous spread
2. Contiguous spread (e.g. extension of sphenoid sinusitis into cavernous sinus, subarachnoid space = septic intracranial thrombophlebitis) 3. Neuronal transmission (e.g. reactivation/spread of HSV type 2) |
|
|
What are the 3 general things that lead to the mechanism of injury in meningitis?
|
1. ↑ BBB permeability = vasogenic edema
2. ↑ CSF outflow resistance (at arachnoid granulations) = interstitial edema (hydrocephalus) 3. ↓ cellular metabolism = cytotoxic edema = ↑ ICP |
|
|
What is aseptic meningitis?
|
Any meningitis, the cause of which is not apparent after initial evaluation and stains and cultures of CSF
Usually viral, but can be bacteria, systemic illnesses, ibuprofen |
|
|
What is subacute/chronic meningitis?
|
Meningitis while milder symptoms presenting over a few days (not 24 hours)
TB Cryptocccus Siphilis |
|
|
What causes fever + focal encephalitis?
|
HSV1
• Ascends via retrograde transneuronal spread • Acute necrotizing encephalitis |
|
|
What pathogens, other than HSV-1, access the CNS by neuronal transport to cause encephalitis?
|
Rabies
Amoeba (naegleria fowleri, through cribriform plate) |
|
|
What disease may present with flaccid paralysis in addition to encephalitis?
|
West nile - polio-like symptoms
|
|
|
What is cercopithecine herpesvirus?
|
Virus in monkeys
Causes fatal ascending myelitis Treatable with valacyclovir |

