![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
123 Cards in this Set
- Front
- Back
|
What are the 4 key features of primary neoplasms?
|
1. Pt age
2. Tumor Location 3. Histologic Type of Tumor 4. Histologic Grade |
|
|
Pediatric CNS neoplasms differ in type and location from adult CNS neoplasm
|
i. In children, brain tumors are the most common form of solid tumor (leukemia, the most common neoplasm in children, are not considered “solid” tumors)
ii. CNS neoplasms are among the top five causes of cancer-related deaths in children and young adults (<40 yo), but account for less than 3% of cancer-related deaths in older adults (older patients die more frequently from cancer, but non-CNS cancer-related deaths predominate by far) |
|
|
Metastatic tumors account for as much as 50% of adult CNS tumors, whereas metastases to the CNS are rare in children
|
IMP***
Where do most CNS tumors occur? Pediatric intracranial CNS tumors are most often in the posterior fossa; Adult tumors are most often supratentorial, involving the cerebrum |
|
|
Who (lol) determined how CNS tumors are graded?
|
WHO tumor grading corresponds to low-grade (grade I or II) and high-grade (grade III and IV) tumors
|
|
|
What are characterisitcs of Benign tumors?
|
Benign tumors, which generally have restricted, localized growth and negligible propensity to metastasize; are relatively well-circumscribed (discrete, smooth boundary between tumor and surrounding non-neoplastic parenchymal and stromal tissue)
|
|
|
Why do we NOT call tumors of the CNS benign or malignant.....at least most neurologists don't
|
Neoplastic cells in the brain, therefore, may more readily infiltrate the surrounding tissue, sometimes making it more difficult to create a “clean margin” of complete surgical excision. Even for relatively well-circumscribed CNS tumors, isolated tumor cells may have infiltrated the brain parenchyma beyond the margin of surgical excision.
Therefore, many pathologists and neurosurgeons prefer the term “low-grade” rather than “benign”, even when referring to slow-growing brain tumors that normally would have a very high likelihood of being surgically cured. |
|
|
What is:
Glioblastoma multiforme |
(GBM) either arises de novo (referred to as a primary GBM) or develops from the progression of lower-grade astrocytomas (referred to as a secondary GBM).
|
|
|
What is Primary GBM?
|
). Primary GBMs account for the majority of cases in older people (mean age at Dx 55 years), and present following a short, rapidly escalating history – usually less than 3 months
|
|
|
What are Secondary GBM?
|
Secondary GBMs typically develop in younger patients (age less than 45 years at Dx) with malignant progression of WHO grade II or III astrocytomas to the WHO grade IV GBM.
The time for progression from the lower-grade astrocytic neoplasm to GBM varies, ranging from 1 to ~ 10 years. There is molecular evidence that primary and secondary GBMs, while morphologically indistinguishable, represent distinct tumor entities |
|
|
Primary GBMs follow a progression of acquired genetic mutations beginning with EGFR amplification or overexpression, followed by alterations in MDM2 and p16 gene expression, PTEN mutation, and RB alterations.
|
Secondary GBMs, on the other hand, exhibit p53 mutations and PDGR-A or PDGF- overexpression early, followed by RB alterations and PTEN mutations
The amplification of EGFR gene in primary GBMs has led to the use of cetuximab, a specific monoclonal antibody that binds to and thereby inhibits downstream activation of the EGFR receptor, in the treatment of some GBMs. |
|
|
Before we really tactile Leonard's stuff let's review some of the cells for the PNS and CNS
|
First Aid Style
|
|
|
How does the Nervous system develop embryologically?
|
neuroectoderm: CNS tumors, ependymal cells, oligodendroglia, astrocytes
Neural Crest: Schwann cells, PNS neurons Mesoderm: Microglia --> Macrophage like |
|
|
What cells are:
Neurons |
permanent cells
do not divide large cells with prominant nuclei Nissle substance in cell body, dendrites |
|
|
What cells are:
Astrocytes |
Physical support
repair cells K metabolism removal of excess neurotransmitters maintains BBB reactive gliosis in repsonse to injury GFAP is cell marker |
|
|
What cells are:
Microglia |
CNS phagocytes
mesodermal origin respond to tissue damage by becoming large phagocytic cells |
|
|
What cells are:
Oligodendroglia? |
myelinates multiple CNS axons (30)
predominant in WHITE matter Destroyed in Multiple Sclerosis Look like fried egg on H&E |
|
|
What cells are:
Schwann cells |
Myelinates only 1 axon in PNS
promotes axonal regeneration derived from neural crest cells destroyed in Guillain-Barre syndrome Aucustic neuroma --> type of schwannoma, typically located in internal acoustic meatus (CN VIII) |
|
|
We will now begin tumors of the CNS
|
Again questions you need to ask yourself:
Presenting signs and symptoms (S/Sx’s & P.E.) Patient age (Hx) Location (P.E. & radiology) Imaging features (radiology) Histologic appearance (pathology) **In general, this is the primary predictor of biologic behavior (prognosis) --> appropriate treatment |
|
|
Objective:
Compare and Constrast Generalized and Localized presenting signs and symptoms of CNS tumors |
Generalized: No gain or loss of function specific to a discrete neuronal sensory, motor, or processing system; “diffuse” effects due to increased intracranial pressure (ICP), metabolic and/or electrochemical disturbances
Focal Deficits: based upon specific location of mass or destructive effect |
|
|
What lobe is affected?
altered cognition, personality changes, expressive aphasia (esp. the posterior left anterior frontal gyrus); motor disturbances (esp. the precentral gyrus --> contrlateral deficits) |
Frontal Lobe
|
|
|
What lobe is affected?
seizures; emotional changes; receptive aphasia |
Temporal lobe
|
|
|
What lobe is affected?
sensation abnormalities (contralateral) cortical-type: impaired appreciation of shape, size, weight, texture (astereognosia) thalamic syndrome: spontaneous pain |
Parietal lobe
|
|
|
What lobe is affected?
partial visual field deficits (cross homonymous hemianopia) |
Occipital lobe
The examples of lobular lesions are focalized or localized examples |
|
|
Memorize this slide!
|
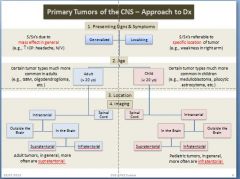
|
|
|
Know this slide
|
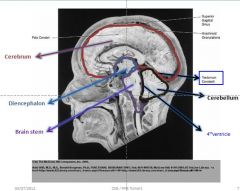
|
|
|
Just to make things clear....
most comon tumors in 1. adults 2. childre |
Adult (>20y.o)
Certain tumor types much more common in adults (e.g., GBM, oligodendroglioma, etc.) Child (<20) Certain tumor types much more common in children (e.g., medulloblastoma, pilocytic astrocytoma, etc.) |
|
|
In general are adult tumors more likely to be Supratentorial or Infratentorial?
|
Supratentorial
|
|
|
In general are child tumors more likely to be Supratentorial or Infratentorial?
|
Infatentorial
|
|
|
what are generalized tumors due to mostly?
|
Mass effects in general
increased ICP: headache, N/V |
|
|
What er Localized tumors usually due to?
|
S/Sx’s referable to
specific location of tumor (e.g., weakness in right arm) |
|
|
What is cerebral edema?
|
Cerebral edema = increase in “brain water”
|
|
|
What are the 3 types of cerebral edema?
|
1. vasogenic
2. cytotoxic 3. interstitial |
|
|
What is vasogenic edema?
|
increased BB permeability --> extracellular***
Damaged vessels: inflammation, neovascularization White matter > gray matter; steroids helpful |
|
|
What is cytotoxic edema?
|
impaired Na/K ATPase --> intracellular***
Hypoxia/ischemia; intact BBB Gray matter = white matter; steroids not helpful |
|
|
What is Interstitial edema?
|
transependymal fluid shift --> extracellular***
Due to increased CSF pressure (hydrocephalus) |
|
|
what type of radiologic modlaity would you use for GBM?
|
CT + contrast
|
|
|
What type of radiologic modality would you use for Metastatic Adenocarcinoma
|
MRI --> T1 + Contrast
|
|
|
Objective
What cells are present in the CNS that could likely give rise to a neoplastic process? |
Glial Cell: astrocytes, Oligodendrocytes, Ependymal cells, Microglia
Neurons Other neuroepithelial cells: choroid plexus Embryonal (primitive neuroepithelial cells) Cells of the meninges (“arachnoid cap cells”) Hematolymphoid (lymphocytes) Germ cells |
|
|
Which cells of the CNS make up the Neuroepithelial?
|
- Glial cells
- Neurons - Other neuroepithelial cells: choroid plexus - Embryonal (primitive neuroepithelial cells) |
|
|
What are CNS tumors graded on?
|
*Histologic Features
Not really staged (size; extent of local growth or presence of metastasis) |
|
|
What is the malignancy scale based on?
|
WHO histologic grade: I, II, III, IV (“malignancy scale”)
|
|
|
What is the major determinate for WHO grade?
|
***LOCATION
Location; patient age; neurologic status In general, the WHO grade suggests a given prognosis; though depends as well upon tumor type, location, and pt age) |
|
|
What is Grade 1?
|
Grade I: may be curative with surgery
|
|
|
What is Grade 2?
|
Grade II: typically survive > 5 yrs
|
|
|
What is Grade 3?
|
Grade III: typically survive ~ 2-3 yrs
|
|
|
What is Grade 4?
|
Grade IV: typically < 1 yr survival (though up to 5-6 yrs depending upon type and availability of effective chemotherapy)
|
|
|
So now we will look at the grades and the histologic features of each tumor***
EXAM mofo |
What is WHO grade ?
Low proliferative activity Typically well-circumscribed; good potential for surgical cure |
|
|
WHO grade II
Low proliferative activity Infiltrative; often recur; may progress to higher grade |
WHO grade III
Greater proliferative potential; more aggressive behavior Generally treated with adjuvant chemo/XRT WHO grade IV High proliferative activity; tumor necrosis; “glomeruloid” endothelial proliferation/neovascularization Typically “not curable” |
|
|
What grade is this?
High proliferative activity; tumor necrosis; “glomeruloid” endothelial proliferation/neovascularization Typically “not curable” |
WHO grade IV
|
|
|
what grade is this?
Greater proliferative potential; more aggressive behavior Generally treated with adjuvant chemo/XRT |
WHO grade III
|
|
|
What grade is this?
Low proliferative activity Typically well-circumscribed; good potential for surgical cure |
WHO grade I
|
|
|
What grade is this?
Low proliferative activity Infiltrative; often recur; may progress to higher grade |
WHO grade II
|
|
|
Let's look at age and location of tumors for adults and children
|
where do most adult tumors occur?
~66-75% supratentorial |
|
|
Where do most child tumors occur?
|
66-75% infratentorial
|
|
|
are adults or children more likely to die from a brain tumor?
|
2nd leading cause of cancer death <15 yo; most common solid tumor for CHILD
Relatively rare cause of cancer death --> ADULTS |
|
|
What are the main CNS tumors seen in adults?
|
1 ° Tumor frequency
~50% astrocytic (cerebrum) ~25% meningiomas |
|
|
What are the main CNS tumors seen in children?
|
1 ° Tumor frequency
~35% astrocytoma Cerebellum: ~20-25% (JPA) Pontine: ~10% ~20-25% medulloblastoma (cerebellum) ~12% ependymoma |
|
|
Are metastatic tumors more common in adults or children?
|
ADULTS: ~ 50% are metastatic (solid organ)
#1 Lung**; breast; melanoma, kidney, GI CHILDREN: Metastases are rare (leukemia) |
|
|
tumor location pic
|
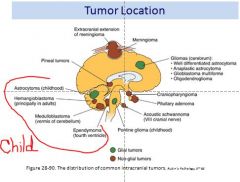
|
|
|
What are CNS tumors Aggressiveness (malignancy) usually due to
|
Infiltrative growth pattern with local recurrence
Disruption of vital structures in CNS Some lower grade tumors may be more rapidly lethal given their location |
|
|
Do CNS tumors metastasize?
|
Rarely metastasize outside CNS
May “seed” within CNS/meninges via CSF Medulloblastoma; ependymoma |
|
|
Work through a pt presentation
picture |
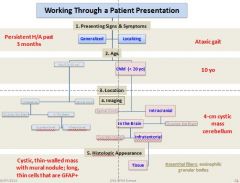
|
|
|
If you have metastese of CNS lesion what will they look like?
|
Most often multiple
Ring-enhancing, sharply demarcated lesions Surrounded by zone of edema Gray-white junction of cerebrum |
|
|
Where are most metastases to the CNS from?
|
#1 Lung
#2 Breast #3 Melanoma Choriocarcinoma ,rare tumor but often w/ mets to brain (testicular germ cell tumor) |
|
|
What are glial cells?
|
non-neuronal cells that maintain homeostasis, form myelin, and provide support and protection for neurons in the brain, and for neurons in other parts of the nervous system such as in the autonomic nervous system.[1] In the human brain, there is roughly one glia for every neuron with a ratio of about two neurons for every glia in the cerebral gray matter.[2]
Some glial cells function primarily as the physical support for neurons |
|
|
What are the 3 main glial cells in the CNS?
|
1. Astrocytes
2.Oligodendrogliomas 3. Ependymomas |
|
|
What is the function of astrocytes?
|
physical support
repair maintenance of BBB |
|
|
What is the funciton of
Oligodendroglia |
Myelinate multiple CNS axons
1 for up to 30 axons make up WHITE matter |
|
|
What is function of
Ependymal cells |
line the cavities of the CNS and make up the walls of the ventricles. These cells create and secrete cerebrospinal fluid(CSF) and beat their cilia to help circulate the CSF and make up the Blood-CSF barrier.
|
|
|
What are the 4 grades of Astrocytomas?
|
WHO grade I: pilocytic astrocytoma (childhood)
WHO grade II: diffuse infiltrating (adult) WHO grade III: anaplastic (adult) WHO grade IV: GBM (adult) |
|
|
What astrocytoma grade is most prevalent in children?
|
WHO grade I: pilocytic astrocytoma (childhood)
|
|
|
What astrocytoma grade is most prevalent in adults?
|
WHO grade II: diffuse infiltrating (adult)
WHO grade III: anaplastic (adult) WHO grade IV: GBM (adult) |
|
|
Pilocytic astrocytoma
|
WHO Grade I
Circumscribed: amenable to surgical extirpation Cystic with mural nodule 86-100% 5 yr survival; One of most common pediatric brain tumors**** Cerebellum; brain stem |
|
|
What will you see microscopically for a Pilocytic astrocytoma
|
Microscopic findings:
Rosenthal fibers*** Microcalcifications (dystrophic calcification) |
|
|
This typical cerebellar example is characterized by a solid, brightly contrast-enhancing mural component and associated cyst on MRI?
|
Pilocytic Astrocytoma
|
|
|
If you see "carrot like" fibers what should you think?
|
Pilocytic Astrocytoma***
Rosenthal fiber (“carrot-like”)** Pilocytic astrocytoma. Varicose Rosenthal fibers lie among the otherwise delicate and hair-like cytoplasmic processes for which the pilocytic astrocytoma is named. |
|
|
Diffuse Infiltrating Astrocytoma - Adult
|
WHO grade II
Fibrillary histologic features most common ~ 15% of astrocytomas; peak ~30-40 y Frontal white matter; disrupts gray-white junction 5 yr survival with total resection + XRT = 70% 50% will progress to higher grade over time |
|
|
What are the microscopic features of Diffuse Infiltrating Astrocytoma - Adult
|
Microscopic:
No mitoses; hemorrhage is relatively rare May see microcalcifications increase in p53 staining (mutated form of p53) |
|
|
generalized expansion and hypodensity of infiltrated regions with only modest mass effect and no foci of bright signal enhancement that would indicate blood–brain barrier disruption on MRI?
Key: Hypodensity....black |
WHO Grade II Astrocytoma
|
|
|
What stain do you use for Grade II Astrocytoma
|
GFAP Stain
Diffuse fibrillary astrocytoma. An immunoperoxidase preparation demonstrates labeling of tumor cell bodies and processes by a monoclonal antibody to glial fibrillary acidic protein (GFAP). |
|
|
What will GFAP positive cells look like?
|
Brown staining indicates GFAP-positivity
GFAP = glial fibrillary acidic protein |
|
|
Anaplastic Astrocytoma - Adult
|
WHO Grade III
Increased cellularity, cytologic atypia (pleomorphism) Mitotic figures are present; +/- endothelial proliferation; no necrosis; rare calcification Immunoperoxidase stains for proteins present in actively cycling cells can indicate growth fraction of tumor (e.g., MIB-1 stain) ~30% of astrocytomas; peak age 40-60 y Median survival ~ 3 yrs |
|
|
Glioblastoma Multiforme (GBM) - Adult****
you will see GBM again |
WHO Grade IV
~ 50% of astrocytomas Most frequent primary brain tumor in adults Peak age: 45-60 y Most common location: deep frontotemporal Median survival: 8-18 months Molecular: = EGFR (Chr. 7)*** Presence of tumor necrosis and pronounced endothelial cell hyperplasia |
|
|
Where do you see primary GBM more commonly?
|
Primary GBM – arise de novo
Younger patients***EXAM Due to epidermal growth factor receptor mutations/amplifications early |
|
|
Where are secondary GBM found?
|
Secondary GBM – arise via progression from lower-grade glioma
Older patients*** p53 mutations early & increased PDGF-A receptor signaling late PDGF-A = platelet-derived growth factor-A |
|
|
What do GBM look like on radiographs?
|
, many glioblastomas are characterized by a bright (“enhancing”) ring (representing intact, abnormally vascularized tumor tissue in which the blood–brain barrier is disrupted) that surrounds a region of hypodensity (central necrosis).
|
|
|
What will you see histologically with GBM?
|
GBM – Palisading Necrosis****
. Dense cellularity, striking pleomorphism, and zones of coagulative necrosis lined by “palisading” tumor cells characterize the prototypical glioblastoma. |
|
|
What happens to the GBM cells?
|
Endothelial Hyperplasia***
Note the complex, “glomeruloid” quality of the microvascular |
|
|
Oligodendroglial Tumors
|
WHO Grade II - oligodendroglioma****
WHO grade III – anaplastic ~10% gliomas; peak age 35-40 y Often mixed with astrocytoma (mixed oligoastrocytoma) Overall 5 yr survival typically better than for pure astrocytomas (grade-for-grade) Tumors with chromosome 1p/19q deletions particularly susceptible to chemo (PVC)**** |
|
|
What is the micro of Oligodendroglial Tumors
|
Micro:
“Chicken-wire” vasculature; “fried egg” cytology; calcifications (~80%) Uniform, round nuclei and clear perinuclear halos (artefacts of delayed fixation) typify well-differentiated oligodendrogliomas |
|
|
Ependymoma
|
Ependymomas can be WHO grade I, II, or III (in the I-IV WHO grading system).
< 5-9% of 1° CNS tumors Bimodal age distribution: peak ages 1-5 y and <35 y Location: Children 4th ventricle; lateral ventricles Adults Spinal cord; 4th ventricle |
|
|
What is a variant of Ependymoma?
|
myxopapillary (possible surgical cure)
Myxopapillary ependymomas are WHO grade I**** |
|
|
What is the micro for Ependymoma
|
Microscopic: true and pseudorosettes
******Ependymoma - Rosettes |
|
|
What are true rosettes?
|
The true ependymal rosette contains a well-defined central lumen
|
|
|
What are pseudorosettes?
|
cytoplasmic processes of ependymal tumor cells condense about blood vessels to form pseudorosettes.
|
|
|
what are you Choroid plexus tumors?
|
1. Papilloma - benign
2. Carcinoma - malignant |
|
|
Choroid Plexus Tumors
Papilloma (benign) |
Children > adults
Children: lateral ventricles most common Adults: 4th ventricle most common May cause a noncommunicating hydrocephalus |
|
|
Choroid Plexus Tumors
Carcinoma (malignant) |
Very rare
Most often in children |
|
|
Medulloblastoma
|
WHO grade IV (highly aggressive)
Derived from “medulloblast” – immature cells that can become glia or neurons Sometimes categorized with other embryonal tumors, termed PNET (primitive neuroectodermal tumor) Small, round(ish) blue cells |
|
|
Where are Medulloblastoma most commonly found?
|
One of most common CNS tumors in children --> cerebellum****
EXAM Frequently disseminate in CSF; 50% metastasize 5 yr survival = >50% with surgery, chemo, XRT |
|
|
Micro for medulloblasoma
|
Micro:
Rosettes; pseudorosettes Homer Wright rosettes ****EXAM Homer Wright rosettes consist of tumor cell nuclei disposed in circular fashion about tangled cytoplasmic processes. These structures are indicative of differentiation along neuronal lines. Medulloblastoma with cellular enlargement, often prominent nucleoli, and pronounced mitotic and apoptotic activity are features of this aggressive tumor. |
|
|
Meningioma
|
Generalized or localizing presentation
~ 15% of primary intracranial tumors; peak age 40-60 y 90% cranial; 9% spinal; 1% ectopic Monosomy chromosome 22 (22q12 = NF2 gene --> merlin)**** Incidence increased following radiation and in people with NF2 Arise from arachnoid cap cells (meningothelial cells) Well demarcated, firm/rubbery |
|
|
How are Meningiomas graded?
|
WHO grade I (>90%): cured with surgical extirpation
WHO grade II (6%): atypical*** (hypercellularity; frequent mitoses; necrosis); variants 5 yr survival: 30%; 50% recur within 1.5 yr; 5% metastasize WHO grade III (2%): anaplastic**** (brain invasion, metastasis); variants |
|
|
Meningioma radiograph
|
Circumscription, homogeneous contrast enhancement and anchorage to the dura
demonstrates thickened and abnormally enhancing dural “tails” extending from the lesional borders |
|
|
Primary CNS Lymphoma
|
Uncommon*****
~ 2% of extranodal lymphomas; 1% of intracranial tumors Except in immunocompromised patients Most are of B-cell origin***** EBV-positive Most often high-grade and relatively poorly responsive to chemotherapy May respond initially to corticosteroids Typically more responsive to radiation tx |
|
|
Germ Cell Tumors CNS
|
Primary to brain vs. metastasis
Primary Midline --> pineal, suprasellar regions Primary brain Germinoma = testicular Seminoma******EXAM Tumor markers and XRT/Chemo responsiveness similar to peripheral correlates |
|
|
Tumors of the PNS......
What are the PNS Peripheral nerve sheath tumors? |
Neuromas (non-neoplastic)
Schwannomas Neurofibromas Malignant peripheral nerve sheath tumors (MPNST) |
|
|
What are the 2 PNS tumor syndromes
|
NF1
& NF2 |
|
|
What is a PNS Neuroma?
|
Neuroma (traumatic neuroma)
Tangled mass of axons, reactive proliferations of Schwann cells, fibroblasts*** Painful (e.g., Morton’s neuroma) NOT neoplastic |
|
|
How are Schwannomas graded?
|
Schwannoma (WHO grade I)
|
|
|
How are Neurofibroma graded?
|
Neurofibroma (WHO grade I)
|
|
|
How are Malignant peripheral nerve sheath tumor (MPNST) graded?
|
Malignant peripheral nerve sheath tumor (MPNST, WHO grade III or IV)
|
|
|
Schwannoma
|
In general, occur as solitary tumors, firm and encapsulated***
Proliferation of neoplastic Schwann cells can be surgically separated from associated nerve successfully In NF2***, multiple Schwannomas Bilateral vestibular Schwannomas Deletion/mutations in NF2 gene, 22q12**** How would such patients likely present? DEAF |
|
|
What does NF2 gene, 22q12 code for?
|
Protein Merlin
|
|
|
Schwannoma
Characteristic histologic feature |
Antoni A** (dense fibrillary) tissue
Verocay bodies**: palisades of elongated, bipolar cells around pink fibrillary stroma Antoni B** (loose reticulated) tissue Note the more cellular "Antoni A" pattern on the left with palisading nuclei surrounding pink areas (Verocay bodies, circle). "Antoni B" pattern with a looser stroma, fewer cells, and myxoid change. |
|
|
Neurofibromatosis
|
Neurofibromas
Composed of Schwann cells, fibroblasts, collagen, reticulin Unencapsulated** and infiltrate nerves***; not thought to occur intracranially Cannot easily surgically remove neoplasm without damage to nerve |
|
|
Neurofibromatosis Type 1
|
NF1 (von Recklinghausen’s NF) – peripheral*****
Accounts for ~ 90% of NF AD, but ~ 50% occur by spontaneous mutations without a family Hx; early onset Ch 17**** 33% have mental retardation ~ 20% have CNS lesions (e.g., spinal astrocytomas) |
|
|
NF1 – a Phakomatosis
|
Inclusion criteria – at least 2 of the following:
Six café au lait spots Two neurofibromas One plexiform neurofibroma Axillary or inguinal freckling Osseous lesion (e.g., sphenoid dysplasia) Optic glioma Two Lisch nodules (iris hamartomas – only seen in NF1) Relative with NF1 |
|
|
Neurofibromatosis Type 2
|
NF2 – central type
Less common, with later onset***** AD Ch22***** Associated with bilateral vestibular Schwannomas, meningiomas**** |
|
|
MPNST
|
Arise de novo or via progression of nerve sheath tumors******
Neurofibromas, possibly Schwannomas ~ 10-15% of neurofibromas in NF1 become malignant ~ 50% of people with a MPNST have NF1*** ~ 75% recur and cause death |
|
|
Paraneoplastic Syndromes
|
Can be related to CNS or PNS tumors
Autoimmune or viral (possibly): immune rection against tumor antigens cross-react with CNS or PNS antigens Lambert-Eaton syndrome**** Antibodies against presynaptic voltage-gated Ca2+ channels Small cell lung Ca |
|
|
Limbic encephalitis: subacute dementia
Small cell lung carcinoma |
Cerebellar degeneration
Destruction of Purkinje cells -->Anti-Yo antibodies Breast, ovarian carcinoma Sensory neuropathy: Anti-Hu antibodies (small cell lung Ca, lymphoma) |
|
|
Paraneoplastic Syndromes
|
Lambert-Eaton syndrome
Limbic encephalitis: subacute dementia Cerebellar degeneration Sensory neuropathy |

