![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
271 Cards in this Set
- Front
- Back
|
Examples of Electrical Synapse
|
Fast Spiking Pyramidal Cortical Neurons
Crayfish Escape Reflex |
|
|
Different types of Chemical signals in synapses and their differences (x3)
|
Neurotransmitters<modulators<hormones
Lower In terms of speed of transmission, response time. Also higher ones modulate rather than transmit info |
|
|
Hemichannels
|
Connexin hemi channel in either membrane = gap junction. Transmit in either direction. Sync electrical activity
|
|
|
Ionotropic vs Metabotropic synaptic action
|
Ion channels vs GPCR. GPCRs also presynaptic. GPCRs can directly activate Ion Channels, or activate 2ary Cascades
|
|
|
Advantage of Transmitters (vs electrical synapses)
|
Plasticity (LTP/LTD)
|
|
|
Examples of Amino Acid Transmitters
|
Glutamate, gabba-aminobutyric acid, Glycine
|
|
|
Examples of Biogenic Amines
|
Catecholamines: Dopamine, Noradrenaline, adrenaline
5-HT, Ach |
|
|
Examples of Neuropeptides
|
Opioids, Pituitary, Insulin, Tachykinins
|
|
|
Gaseotransmitters
|
CO, NO -> not stored, released when synthesised
|
|
|
Difference in synthesis between small and large NTs/NMs (neuromodulators)
|
Large ones (e.g. peptides) made in cell body, small ones translated in terminal
|
|
|
Loading of Transmitters into Vesicles
|
Proton Pumps make synapse acidic, then cotransport H+(out)/NT(in) = 2ary Active Transport
|
|
|
Inactive Vesicle Storage
|
Stored by Synapsin Actin, helped by piccolo/bassoon molecules
|
|
|
Priming for Vesicle Release
|
Synaptobrevin (V-snare) spontaneously zips with snap-25, Syntaxin (T-snares). Munc Mediated control of zipping in active zone (and not before)
|
|
|
Calcium sensor mechanism in synaptic fusion
|
Synaptotagmin acts as Ca2+ sensor, displaces complexin from blocking fusion -> allows fusion. Then
|
|
|
Unzipping of vesicles
|
NSF mediated, although difficult to understand, because SNAPS must be inactivated to prevent rezipping.
|
|
|
2ary messengers of GPCRs
|
Adenylyl Cyclase = ATP -> cAMP -> activates PKA
Phospholipase C = PIP2 -> IP3 -> releases Ca2+ -> DAG -> activates PKC |
|
|
Fates of Transmitter in Cleft
|
Diffusion away, reuptake (e.g. Astrocyte glia remove glutamate -> reduces seizures), enzymatic breakdown (e.g. ACh esterase). Depending on rate of removal -> high fidelity, but can be blocked e.g. cocaine blocks Dopamine, serotonin, noradrenaline reuptake
|
|
|
Which GPCR TM segments change
|
3 and 6 reorient
|
|
|
What happens when GPCR Activated
|
G protein alpha exchanges GDP for GTP and dissociates beta gamma, both can have downstream effects. GTP hydrolysed eventually. Cascades show divergence and convergence.
Can signal via non-G protein pathway |
|
|
Significance of GPCRs in pharmacology?
|
50-70% drugs target them
|
|
|
Types of GPCR
|
850, with 500 olfactory.
Non olfactory - rhodopsins, metabotropic glutamate, secretin/calcitonin-like, smoothened/frazzled-like, adhesion receptors. Differ in Agonist binding, 3rd Intracellular loop (binds G Protein), N-C terminals |
|
|
Ensemble Theory
|
GPCRs continuously oscillate between configurations with different properties, affected by ligands. (supported by crystal structures)
|
|
|
Dimer formation of GPCRs
|
Homo or Hetero: Glutamate receptors use dimers or monomers, allosteric modulators only modulate dimers.
GABAb receptors - between B1 (binds GABA) B2 subunits (binds G Protein). |
|
|
Agonist specific Coupling
|
Different Agonists can bind GPCRs - different receptor coupling. E.g. mu opioid receptor affected differently by lots of different ligands
|
|
|
Desensitization/Internalisation of GPCRs
|
Phosphorylation by GPCR kinase, Beta arrestin binding. Or PKA mediated phosphorylation -> may also switch paths.
Can lead to internalisation |
|
|
Cotransmission
|
Multiple neurotransmitters in one synapse - usually:
one Fast transmitter from one neuron, Modulatory NTs + ATP from another neuron. Generally neurons use same NTs in all axons |
|
|
3 criteria for NT
|
Present in specific neurons (stored in Vesicles)
Ca2+ dependent release Receptors on Post Synapse (i.e. if added -> at least partial AP) |
|
|
Detecting Neurotransmitters
|
Immunochemistry, in situ hybridization
Stimulate electrically/K+ depolarisation -> induce release (but unsure which neuron) Apply NTs iontoporetically (again unsure about which neuron they act on) |
|
|
Glutamate
|
Excitatory
Information processing Made from alpha ketoglutarate + glutamine Binds Mono or Dimer Glutamate GPCRs Binds AMPA/NMDA receptors. Can be taken up by glia in glutamine cycle |
|
|
GABA
|
Inhibitory (except in development)
Affects Cl-/K+transporters Also can be reuptaken by glial cells Binds GABAa ionic receptors and GABAb metabotropic receptor dimers |
|
|
Peptide transmitters
|
Modulatory and can be generally affective to brain state e.g. orexin -> wakefulness
|
|
|
Noradrenaline / Dopamine / 5-HT / Histamine
|
Noradrenaline - attention, arousal, sleep wake
Serotonin - sleep-wake, mood, perception Histamine - Arousal, Energy metabolism, 'general effects' Dopamine - voluntary movement, reward/addiction |
|
|
Glia functions
|
Uptake NTs (e.g. Glutamate)
Metabolic support (e.g. convert glucose to lactose) Coordinate (eg. NG2+ can control local neurons through AP activity) Modulate (e.g. release of D-serine = coactivator of glutamatergic NMDA receptors = gliatransmitters) |
|
|
Myasthenia Gravis: disease, symptoms, treatment
|
up to 1:20k people. Antibodies inactivate ACh receptors.
Inability to contract properly - droopy eyelids Neostigmine prevents AChE working |
|
|
Sleep disorders: prevalence/cost, natural effectors, treatment
|
1:4, up to $100b in US.
Natural factors: SCN circadic clock, internal environs, emotion (orexin mediated) Prevent wakefulness = antihistimine, GABA enhance (e.g. benzodiazepines) Encourage wakefulness - noradrenaline (e.g. amphetamines block reuptake |
|
|
Sleep systems normally
|
Raphe (5-HT), TMN (Histamine), LC (Noradrenaline), Hypothalamus (Orexins) -> Wake
VLPO (GABA) neurons -> inhibit wake |
|
|
Parkinson's: symptoms, cause, treatment (x5)
|
Movement disorder = struggle with voluntary. Dopaminergic neurons disrupted, so balance shifts to involuntary.
Treatment: L-Dopa (but adaptation - dopa holidays), block MAO-mediated degradation, dopamine alts e.g. bromocriptine, implants of fetal substantia nigra, deep brain stimulation |
|
|
Epilepsy: symptoms, cause, treatment
|
Seizures/convulsions, brain storm
Neurons all firing in synchrony. Calcium buildup in Glia? So Target GABA? Enhance GABAa receptor activation (benzos) Inhibit GABA transaminase enzyme (vigabatrin) Block reuptake of GABA (tiagbine) |
|
|
3 Parameters of Sound
|
Frequency (pitch), Amplitude (volume), Phase (harmonic detection etc.)
|
|
|
dB SPL =
|
20log(pressure/20microPa). Used because range 10^12:1
|
|
|
Band Pass Filters
|
Low pass - cut off high, high pass - cut off low, band pass - cut off both ends.
|
|
|
EAM
|
Open ended tube - resonant peak -> gain at speech frequency
|
|
|
Pinna
|
Shape of ear - helps localisation. If changed with mould - prevents localisation, but relearns
|
|
|
Middle Ear anatomy
|
Ossicles (malleus, incus, stapes) + MEMs
|
|
|
Functions of the inner ear
|
Impedance Matching, Loud Sound protection, Antimasking
|
|
|
Impedance Matching
|
Air and Cochlear fluids -> different densities
|
|
|
Loud Sound Protection
|
MEM reflex -> muffles loud sounds. Maybe controlled by medial olivocochlear efferent
|
|
|
AntiMasking
|
MEMs attenuate low frequencies preferentially, lets higher frequencies through
|
|
|
Rinne Test
|
Test for conductive vs sensorineural hearing loss. Place tuning Fork on Mastoid or EAM - see which is louder (should be air conduction). Also Weber Test (similar but diff positions) - double check.
|
|
|
IHCs vs OHCs (anatomy)
|
1 row IHC w/ 90% nerve fibres (type I myelinated)
3 rows of OHCs w/ 10% nerve fibres (type II unmyelinated) |
|
|
Three different fluids in cochlea
|
Scala vestibuli, media, tympani
|
|
|
Basilar Membrane Tuning +evidence (x4)
|
Sharply tuned w/OHC mediated active negative feedback.
Evidenced by Cochlear Vulnerability, Otacoustic Emission, OHC interruption changes IHC response, Passive Models fail |
|
|
Transduction mechanism in hearing
|
Physiological displacement of Stereocilia = bundles of not-quite cilia. Shifts Tip-Links, displaced towards largest stereocilia -> excitatory flow of K+ in depolarises.
|
|
|
Scala Media Endolymph
|
+100mV rich in K+, flows into -60mV hair cell
|
|
|
IHC vs OHC dB
|
IHCs>60dB, OHC<60dB.
but OHC only secondary i.e. loss -> weakens <60dB, IHC loss -> total loss |
|
|
Frequency Coding (audio)
|
Overlapping parallel band pass (measured by Q factor) -> characteristic frequency = most sensitive part of filter
|
|
|
Temporal Coding (audio)
|
Phase locking - match firing rate to frequency of sound, but 1kHz firing limit
up to 8kHz phase lock (barn owl) -> volley principle |
|
|
Intensity Coding (audio)
|
3 types of neurons with different spontaneous discharge rate (depending on threshold for firing). Combined to get full 140dB dynamic range
|
|
|
Two tone suppression
|
Regions above and below excitatory response inhibited at BM level (too fast for descending system)
|
|
|
Superior Olive - > cochlea
|
Olivocochlear descending fibres
From Lateral superior olive: Unknown purpose From Medial superior olive: to OHCs -> loud noise protection/own voice i.e. controls adaptation to background noise. |
|
|
Cochlear Nucleus function
|
Retina of auditory system. Held Bulb end synapse = largest in brain.
Parallel processing segregates timing, intensity, frequency information, but also serial processing of information |
|
|
Interaural Intensity Difference
|
More distant ear shadowed for >1kHz frequency. up to 20dB
Detected by Nucleus Angularis, collated in Posterior Lemniscal Nucleus |
|
|
Auditory Midbrain
|
Space map from the nucleus laminaris and posterior lemniscal nucleus.
|
|
|
Interaural Time Difference
|
Max is 660microseconds, min is 10microseconds.
Detected by Nucleus Magnocellularis, collated by delay lines in nucleus laminaris |
|
|
Light wave basic properties (x4)
|
amplitude, freq, phase, polarisation
|
|
|
Visible wavelengths/intensities
|
300-700nm
10^10-10^20 (photons/m2sr1s1 |
|
|
Albedos
|
Unit of reflectivity between 0.02 - 0.98
|
|
|
What can materials do with light (x5)
|
transmit, refract, reflect, absorb, transduction
|
|
|
Importance of eyes (evolutionarily)
|
Independent evolution >20 times
1/3 cortex in humans, 1/2 in fly |
|
|
Sensitivity vs intensity
|
Reciprocal -> constant response.
i.e. if less sensitive, need higher intensity to see same thing |
|
|
Constant Response (vision)
|
Used to determine components' performance in a system by seeing what stimulus needed to give same response.
e.g. looking at different sensory filters response (e.g. differently tuned rhodopsins) |
|
|
Components that determine spatial resolution in a focusing optical system + how to measure spatial resolution
|
Receptor Spacing, Optical Blur, Photon Noise.
Measure this resolution by sinusoidal grating (contrast sensitivity function) |
|
|
Receptor Spacing (vision)
|
Determined by angle between samplers (e.g. cones) = deltaPHI, so maximum resolvable wavelength = 2xdeltaPHI.
Depends on receptor diameter (min=2microns, although cones can be 0.5-4microns) and focal length, e.g. sparrow's long eyes |
|
|
Optical Blur
|
Sensitivity decreases as spatial frequency gets higher i.e. slimmer lines , because light bleeds from bright to dark = optical point spread function.
Happens because of diffraction, poor focus, lens aberrations |
|
|
Photon Noise
|
Molecular absorption of photons = stochastic according to heisenberg's uncertainty principle, detectors obey square root law. Limits sensitivity because has to be statistically significant number, otherwise eigengrau
Pool over Time/Area, or open lens for more photons |
|
|
How to increase spatial resolution
|
Open lens - lower area of sampling, so higher spatial resolution BUT more peripheral rays (blurs), so trade between resolution and noise.
Make lens further back/slimmer receptors - e.g. sparrow, reduces angular spacing |
|
|
Sensitivity in diff background lights
|
Less sensitive in high background lights (i.e. reduced minimum detectable increase) because contrast coding, i.e. divide by mean background signal using centre surround lateral inhibition. In fact sensitivity proportional to 1/background light (I).
Except bottoms out because of eigengrau + low frequency roll off (i.e. if low spatial frequency, falls on both parts of centre surrounds of RGCs) |
|
|
Eigengrau
|
Spontaneous photon signals by rods, i.e. Hecht (1942) showed that 80 photons/second = threshold for 500 rods, so each rod needs 1photon/6seconds.
Below this eigengrau because spontaneous isomerisation. Finnish frogs show thermally dependant (searching for prey at diff temps). |
|
|
Range Fractionation
|
Scotopic Rods -> Photopic cones
|
|
|
Solutions used by phototransduction to code and transmit spatial, temporal and spectral distribution of light at different I (background lights)
|
Amplification, Adaptation
|
|
|
Structure of Rods/Cones
|
Light Guide, Transduction machinery, Output Synapses (eg. cone pedicles in synapses)
|
|
|
Activation of Rod
|
Photon activates rhodopsin -> Rh* -> GPCR -> guanylate cyclase (ciliary), phospholipase c (rhabdomeric). All bits mapped to cytoskeleton for reliability
|
|
|
Rhabdomeric vs Ciliary
|
Rhabdomeric photoreceptors in compound eyes of arthropods (high SA because folded on top like bart's hair), ciliary in vertebrate (folded to the side like disks)
|
|
|
Amplification and Adaptation in Rods
|
One Rh* can open up many channels, but flexible
Also Rh* mopped up quicker if high light i.e. better temporal acuity (albeit lower sensitivity). i.e. faster cycling at high light (controlled by Calcium) |
|
|
Intensity Coding in Photoreceptors
|
Coded as membrane potential according to R/logI curve (i.e. shifted by adaptation)
|
|
|
Rods and Cones different
|
Rods bind chromophore tighter, so it's more stable and higher sensitivity, but lower temporal accuity
|
|
|
Convergence in Visual System (numbers of photoreceptors and RGC)
|
10^7 rods + 3x10^6 cones
converge on 1.5*10^6 RGCs =factor of 10 |
|
|
How many of types of the different retinal cell
|
1-3 horizontal
14 bipolar? 750 amacrine 10-15 rgcs |
|
|
Bipolar cell's different parallel coding
|
Rod bipolars - 50-100
Blue bipolars - S cones Midget bipolar - one L/M cone |
|
|
Features of Bipolar Cells
|
Graded signal in membrane potential (like photoreceptors)
OFF (ionotropic) or ON (metabotropic) -> establish separate channels with RGCs Sum inputs in adaptation to background light |
|
|
How to adapt visual system to lower light
|
Mop up activated chromophore slower/ bind less tight -> increase sensitivity (but less temporal acuity, i.e. summing over T)
Bipolar cells sum fewer photoreceptors (i.e. sum over A) Horizontal Cells also aid adaptation, by subtracting mean background I (intensity) |
|
|
Cone pedicles
|
Release Glutamate Transmitter at >50 presynaptic ribbons. Invaginated post synapses all ON, so some flat must be OFF
|
|
|
Retinal Ganglion Cells' receptive fields
|
Elliptical/circular w/ centre-surround (mediated by amacrine), but varies in size depending on inputs.
Unique RGCs -> parallel signals |
|
|
Starlight/Twilight Circuit
|
ON1 rod bipolars -> AII amacrine -> ON cone bipolar -> ON rgc
ON2 rod bipolar -> electrical synapse w/ cone -> ON cone bipolar->ON rgc. ON1 circuit sums because of A2 amacrine. Neuromodulators shut down ON1 if high I |
|
|
Foveal transmission
|
Foveal cone has ON and OFF line to brain (midget bipolars/rgc) -> code colour, connect M/L. -> high acuity, but dense midgets
|
|
|
P vs M rgcs
|
P=parvocellular destined MIDGET rgcs -> code colour/form
M=magnocellular destined Parasol rgcs -> coarse sampling, but faster response |
|
|
Why have ON and OFF parallels to brain?
|
increased dynamic range in coding?
more economical on spikes |
|
|
Non uniform sampling of vision
|
Fovea - highest density. Overall acuity follows spacing of P rgcs = matches optic flow of forwar locomotion
Rabbits -> high on edge to pick out predators + on horizon |
|
|
Lateral Geniculate nucleus anatomy/function
|
2 magnocellular, 4 parvocellular layers, lots of small konicellular layer for S colour
Has circular/symmetric ON/OFF like rgc - not simply relay station. Also descending control of eye vergence, focus |
|
|
V1 anatomy/plasticity
|
40 visuocortical areas
M path -> Dorsal Parietal 'where' stream P path -> ventral temporal 'what' stream Hypercolumns with segments that select for orientation, different eyes for each one => economises wiring with similar neurons together Thinner stripes if one eye occluded -> plasticity |
|
|
Mapping of visual space in V1
|
according to rgc density i.e. central 10degrees occupies 50% of V1 +
|
|
|
Selectivity of input from V1 upwards
|
fire less frequently, showing higher selectivity
|
|
|
Significance of Olfaction
|
Phylogenetically old
Behaviourally important - linked to motivation/emotion/feeding/predation |
|
|
Initial Transduction of Olfaction
|
Airborne odorants detected by epithelium (can be turbinated (folded) in rats). Dissolve in mucus film
|
|
|
G[olfactory]
|
Odorant specific GPCRs (c500). Renewed c50 days. Responds to a range of molecules. Adenylyl cyclase based -> opens Na/Ca[cAMP] channels -> depolarise cell / cl- out. Na/Ca exchange (+CaATPase in some species) repolarises
|
|
|
Adaptation to odourants
|
Ca2+/calmodulin adjust sensitivity (2x rapid, one persistent)
|
|
|
Pattern Coding (odour)
|
Each GPCR has a range of mols it responds to (e.g. diff length C chains of aldehyde) - so many have to be compared
|
|
|
Alternative receptors in olfactory system
|
GC-D receptors use guanylate cyclase (natriuretic detection - involved in food prefs etc.)
Vomeronasal receptors use Phospholipase C (pheromones) |
|
|
Beyond the olfactory epithelium
|
Receptor axons pass through cribriform plate to olfactory bulb -> stimulate mitral tufted cells
|
|
|
Cells in Olfactory Bulb
|
Mitral/tufted, periglomerular/granule (lat inhib mediation)
|
|
|
Lateral inhibition in olfactory bulb
|
Contrast enhancement of odorants overlapping patterns
|
|
|
Anterior olfactory nucleus action
|
Inhibits contralateral bulb via anterior commissure with the information from the ipsilateral bulb
|
|
|
Mitral Axon afferents
|
Leave by Lateral olfactory tract -> synapse to olfactory complex x5
|
|
|
Higher olfaction responses
|
more specific to different odourants. overlapping projections, suggest combinatorial coding
|
|
|
Smell in stereo?
|
associated learning with rats (odour, then lick the correct water spout) says yes. humans too (chocolate scented twine)
|
|
|
Vomeronasal olfaction significance
|
projects to amygdala - used to determine gender in rats. If KO - unsure if to show aggression or mating
Humans - trace amine associated receptors -> fertility |
|
|
Tongue anatomy
|
Pappillae = holes
Taste buds= divots in them Taste pores = receptor+supporting cells |
|
|
Projections of the taste pores
|
synapse to chorda tympani, glossopharyngeal nerves
|
|
|
Different Taste types and their receptors
|
Bitter - 30 T2R GPCRs
Sweet/Umami - heterodimeric GPCRs (PLC linked) Sour -> Intracellular acidification (protons block K+v channels) Salt -> Na+ enters via epithelial Na+ leak channels |
|
|
Hot/Cold receptors in taste
|
Capsaicin Receptor TRPV1 (noxious heat -> pain also)
Menthol Receptor TRPM8 |
|
|
Higher paths of taste
|
Receptors -> chorda tympani/glossopharyngeal -> medulla -> thalamus -> 1ary gustatory neocortex -> 2ary (palatability in orbitofrontal) -> amygdala (motivation) + hypothalamus (feeding)
|
|
|
Specific Satiety in taste
|
2ary gustatory cortex (orbitofrontal) modulated by amygdala's motivational paths, sated to components of taste too
|
|
|
Coding in taste
|
Cross fibre, not labelled line -> pattern of activation
|
|
|
Action of Gustatory receptors
|
Bitter, Sweet, Umami all release ATP via gap junction hemichannels (as in electrical synapses), then distance determines their action on afferents.
5HT affects it (sweet, sour) -> gives a specific taste |
|
|
Intero vs Proprio vs Exteroception
|
Intero = sense of organ systems/hunger/internal tates etc
Proprio = sense of muscles/joints Extero = sense of direct interaction with world |
|
|
4 major glabrous receptors
|
Rapidly Adapting = Meissner's Corpuscle (RAI), Pacinian Corpuscle (RAII)
Slowly Adapting = Merkel Cells (SAI), Ruffini endings (SAII) |
|
|
Pacinian Corpuscles anatomy and function
|
layers of lamellae membrane surrounded by fluid - rapid on off. Widely distributed, large field, large size.
Used to detect events through held objects +grip etc. + vibration |
|
|
Meissner's Corpuscles anatomy and function
|
Superficially placed, capsule with schwann wrapped axons. Localised field
Respond to low frequency vibration 50-200hz |
|
|
Merkel Cells anatomy and function
|
Surrounded by keratinocytes, supported by merkel disc, highly localised field
Sensitive to points, edges, curvature up to 0.5mm (braille) - better for dynamic |
|
|
Ruffini endings anatomy and function
|
Axon into fluid filled capsule with collagen, attached to muscles
May perceive motion - specfic. May also be proprioceptive for hand shape (stretch skin -> activate ruffini -> feels like finger flexion) |
|
|
Differences in tactile acuity across body
|
compass test - two point limit.
40mm at shoulder, 2mm at fingers Higher motility -> higher tactile acuity (smaller fields) |
|
|
Other glabrous receptors
|
Thermoreceptors -> free nerve endings (map shows separate modalities for hot/cold, diff proportion depending where) (TRPV1, TRM8). Labelled line.
Nociceptors - also free (Adelta, C). Labelled line. |
|
|
Paradoxical cold
|
Activation of cold receptors at >45C, shows labelled line of those receptors
|
|
|
Aalpha/Abeta vs. Adelta vs C
anatomy and function |
MYELINATION/SIZE: Aalpha/Abeta < Adelta < C
Abeta = Touch / proprioception Adelta = cold, stabbing pain C = warmth, itch, burning pain |
|
|
Effects of Anoxia/Anaesthetic on somatosensory afferents
|
anoxia -> abeta, then adelta
anaesthetic -> C then adelta Used to find theirfuncitons |
|
|
Cauda Equina
|
Top of Spinal Cord
Removal of CSF, or application of anaesthtic |
|
|
Dermatone
|
Area of skin innervated by one dorsal root (boundaries overlap)
|
|
|
Spinal Cord overall anatomy, functional overview
|
31 bilaterally paired nerves - receive sensory afferents, control movements, autonmic innervation
|
|
|
5 major parts of spinal cord (in section)
|
Dorsal column, ventral column = white matter. DCML touch proprioception nerves.
Dorsal Horn, Ventral horn = H shaped gray matter (different lamina). Spinothalamic Pain tract. Lateral Column |
|
|
Central Paths x2
|
Touch proprioception = Dorsal Column - Medial Lemniscal System (DC-ML) (ipsilateral in dorsal column)
Pain = Spinothalamic Tract (contralateral in dorsal/ventral horn) |
|
|
Referred Pain
|
Pain from an internal organ projects to same dorsal horn area -> brain thinks its superficial e.g. angina pectoris
|
|
|
Spinal Cord Lesion types
|
Dorsal Column Lesions (e.g. posterior column syndrome) - loss of DCML (ipsilateral touch)
Central Loss (e.g. syringomyelic syndrome) = loss of spinothalamic tract (contralateral pain) Hemisection (e.g. brown-sequard syndrome) = loss of both (ipsilateral touch, contralateral pain) |
|
|
Trigeminal system
|
Sensory innervation and Motor skills for most of the head. Neuralgia = syndrome where stroking face -> stabbing pain.
|
|
|
Thalamus in somatosensation
|
Can't just be a relay station. Switchboard?
DCML terminates ventral posterior nucleus Spinothalamic terminates ventral medial nucleus (VMPO) |
|
|
Somatosensory cortex (S1) anatomy
|
4 areas (y axis)
6 layers (z axis) - To/from different places (e.g. thalamus to 6, from 4) 300-600micron columns (xaxis) - receives from same place, but different afferents - preserves modality and location |
|
|
Feature detection in S1
|
Certain neurons respond to certain features e.g. direction.
Attention alters responses eg. S-II -> less tactile response with visual task |
|
|
Sensory Homunculus
|
Map of somatosensation in cortex - somatotopic, but overrepresents
|
|
|
Plasticity of Somatosensory cortex
|
e.g. train touch finger for 20 weeks -> 3b of monkey expands
e.g. remove finger - expansion of somatosensory cortex of other finger |
|
|
Phantom Limb
|
Sense of missing limb e.g. map of fingers on upper arm, because the nerves for upper arm have expanded to finger cortex
|
|
|
Definition of pain
|
An unpleasant sensory or emotional experience associated with actual or potential tissue damage
|
|
|
Anterior Cingulate Cortex (pain)
|
Emotional pain - responds to noxious heat and experience, NOT watching pain really
|
|
|
Insula (pain)
|
Homeostatic pain + empathetic + judging pain
|
|
|
Gate control theory
|
Transcutaneous electrical nerve stimulation -> pain relief
Ad/C stimulate T (transmission cells - transmit pain with wide dynamic range) but Ab also connects to T with inhibitory interneurons |
|
|
TENS
|
Transcutaneous electrical nerve stimulation -> pain relief
Ad/C stimulate T (transmission cells - transmit pain with wide dynamic range) but Ab also connects to T with inhibitory interneurons |
|
|
PAG
|
Periaqueductal gray matter of midbrain/raphe nucleus -> mediates descending system of pain. Integrates cortical, thalamic, hypothalamic inputs.
If stimulated -> enough analgesic for abdominal surgery |
|
|
Naloxone effect on Descending Pain
|
Blocks PAG actional by binding opioid receptors -> shows natural opiates being used.
|
|
|
Placebo analgesia specificity experiment
|
Injection of capsaicin , rub placebo on that area. Limb specific. Found to be specific in rats too.
Naloxone intravenously - abolished |
|
|
Difference between pain and nociception
|
Nociception is sufficient but not necessary for pain
|
|
|
Sensitisation of nociceptors + causes x2
|
Painful enough stim -> sensitisation = hyperalgesia + Allodynia also (e.g. pain of touch when sunburnt)
+NGF / Prostglandins - sensitize only |
|
|
Internal factors that can cause pain at free nerve head endings (x3)
|
ATP (from damaged cells)
Bradykinin (Formed by kinin system in inflammation) Acid (released in anoxia / metabolic overload) |
|
|
Arachidonic acid -> ?
|
Prostglandin (PGE2 = sensitizer) made by COXs
|
|
|
Neuropathic Pain? examples x2
|
Nerve Damage that results in lasting (indefinite?) pain. I.e. changes spinal/brain pathways
E.g. Phantom Limb, Diabetic Neuropathy |
|
|
NSAIDs what are they and how do they work?
Examples |
Non Steroidal Anti inflammatory Drugs
Inhibit COXs - so prostglandins can't be made - enters hydrophobic tunnel of COX e.g. aspirin permanently acetylates serine-s30 Aspirin (permanent - inhibit thrombosis) Ibuprofen (Arthritis, gout, soft tissue) Paracetamol (acts on COX-3 in brain, but doesn't really anti inflame, so no |
|
|
Prostglandin - production, blocking, action
|
Made by COXs from Arachidonic Acid
COXs blocked by NSAIDs Act as Vasodilators, so NSAIDs relieve headaches caused by cerebral vasodilation. Plus may facilitate nociceptor neurotransmission |
|
|
Different types of COXs
|
COX-1 constitutive so blocking - side effects
COX-2 induced in inflamed cells so inhibition = anti-inflam/analgesic COX-3 in brain - produces PGs that cause headaches |
|
|
Side effects of blocking COX-1 with NSAIDs
|
gastrointestinal bleeding (PGs inhibit acid secretion)
renal insufficiency (PGs maintain kidney blood flow) stroke (PGs vadilate) myocardial infarction (PGs vadilate) |
|
|
Opioid effects/cascade
Blocked by? |
Morphine like - binds mu, kappa, delta, ORL GPCRs -> activates inward rectifying potassium -> hyperpolarises cell -> inhibits adenylate cyclase -> reduces PG effects
Nalaxone blocks it |
|
|
Examples of Opioids (x4)
|
Morphine
Diamorphine/Heroin (permeates blood brain) Codeine (better for oral absorption + NSAID) Etorphine (1000x more powerful than morphine) |
|
|
Side effects of Opioids (x4)
|
Respiratory depression (mu mediated)
Nausea / Vomiting (mu/delta mediated) Constipation (mu/delta/kappa mediated) Can cause tolerance + dependance |
|
|
Other types of Analgesics apart from Opioids and NSAIDs (x4)
|
Tricyclic Antidepressants (inhibit 5HT uptake = good for neuropathic)
Ketamine (Blocks Glutamatergic nociceptor because NMDA receptor antagonist) Gabapentin (reduces recruitment of subunit of CaV in neuropathic pain) Lidocaine (blocks NaV channels) |
|
|
Motor Cortex location, inputs
|
LOCATION Anterior to central sulcus
INPUTS Senses via thalamus, somatosensory cortex, cerebellar/basal basal ganglia |
|
|
Organisation of the motor cortex
|
Penfield showed a motor map of body but his overlap showed synergistic input, not intersubject variability
BUT brain thinks in movements because muscles activators in more than one place + one cortical neuron could excite many motor neurons =Fractured Somatotopy |
|
|
Fractured Somatotopy
|
The organisation of the motor cortex that's not 1:!, but actually :
Muscles can be innovated by multiple neurons Multiple neurons can innervate muscles |
|
|
Showing the function of the motor cortex
|
Lesion it via a stroke -> spasticity/paralysis
Shows motor cortex in charge of volitional movement |
|
|
What sort of signals does the motor cortex send?
|
Each neuron responsible for direction, so population code (seen by their activation when moving)
+ evart showed that spike frequency proportional to limb force |
|
|
How does a brain machine interface work and what are its problems (x2)
|
10x10 electrode array into cortex -> record movements ->build up a library, then send similar signal
Performance is worse than normal And glial cell build up - protective |
|
|
Plasticity in the motor cortex
|
Maps of Fractured somatotopy initially absent, but motor but gets built up during development.
Map Correlates with skill + complexity |
|
|
What's the Premotor Area, what are its inputs/outputs
|
Humans have 6x premotor/body weight than macaques. Preps for movements. Firing time depends on task complexity. Split into different subdivisions for different movement types
Inputs from prefrontal, parietal, cerebellum, basal ganglia. Outputs to 1ary motor, reticular formation, spinal cord |
|
|
Lesioning the premotor area
|
More subtle deficiencies in developing motor strategy and somatosensory integration failure (e.g. fails to push hand through gap.
Shows it preps for movements. Firing time depends on task complexity. |
|
|
Supplementary Motor Area purpose
|
Imagining Movements i.e. Programming complex sequence + planning/rehearsal + mirror neurons
|
|
|
What are the roles of the motor systems (x5)
|
Move in/Manipulate world
Maintain equilibrium internally Autonomic Communication Sensory |
|
|
Necessity of sensory input for motor control and vice cersa
|
Pseudo Athetosis i.e. holding out arms -> drift
Saccades in vision + kittens moved passively -> development defects |
|
|
3 types of motor movement
|
Reflex - involuntary, few muscles, rapid, stereotyped
Rhythmic - voluntary + reflex, several muscles, stereotyped but modifiable Voluntary - most complex, purposeful, goal directed, initiated by stimuli or motivation, learned + improveable |
|
|
Model system for understanding motor networks
|
Pyloric network of Lobster StomatoGastric Ganglion
Simple, large neurons, few synapses |
|
|
3 levels of organisation in motor system and their relationship
|
Spinal cord, brainstem, higher centres.
Not just top down, CNS can issue commands. Functional Hierarchy though - with planning from the top, execution at bottom |
|
|
Negative feedback vs Feedforward ballistic control in movement
|
Negative feedback delays limit it to slow movements (not catching a ball) -> overcompensate because sensory can't keep up.
Use preprogrammed stored movements (e.g. walking) combined with conditions (e.g. icy) to send movement. ie. set up limb position/muscle tone. But still use proprioceptive feedback in next movements |
|
|
Galen's view of the spinal cord
|
Just a bundle of nerve fibres
|
|
|
Spinal Cord is ....
|
...a flexible system that relays info both ways and generates its own outputs
|
|
|
Where are the motor neurons in the spinal cord
|
Ventral horn (grey matter) - so more grey matter near arms/legs
|
|
|
Proximal Distal rule vs flexor extensor
|
Proximal innervation = more medial
Flexor innervation = more dorsal =Functional Motor Map for motor neurons and premotor interneurons |
|
|
Motor Unit?
|
Motor neuron + fibres innervating muscle = smallest controllable unit BUT muscles can have hundreds of units
|
|
|
3 different types of motor units
|
Slow
Fast Fatigue resistant Fast More tension, but can spend less time in each |
|
|
How to change force output (2 ways)
|
Change the recruitment of motor units OR increase firing rate (limited because fuse eventually)
|
|
|
Locomotor pattern two basic components
How do they change at different speeds |
Swing (flexion) vs Stance (extension)
Less extension at faster speeds, same amount of swing |
|
|
CPG
|
circuits affected by decending tonic input processed in the spinal cord - recruits and coordinates correct motor neurons.
|
|
|
Sherrington CPG suggestion
Brown's Test and the problem with it |
Suggested rhythmic movement caused by reflex chain
Brown tested by transection spinal cord i.e. severs descending, AND cuts sensory dorsal horn - just attached to muscles -> showed stepping when spinal cord stimulated. BUT still may be some sensory input |
|
|
Wilson's Fictive Locomotion and problem with it
|
Completely severed locust spine - showed generation of a flight locomoter pattern when bathed in glutamate
BUT is it applicable to higher animals/vertebrates? |
|
|
Vertebrate CPG (x3)
|
Rat - brain removed shows a jerky, basic movement
Primates - sensory and descending removed -> CPG Humans can't do that so: No descending= Stepping in babies/spinal lesion. No sensory - also some movement. Have to have a very lucky accident. |
|
|
Proposed circuitry for CPG
|
Renshaw Cells + reciprocal inhibitory interneurons -> part of TWO HALF CENTRES -> each control flexor/extensor and mutually inhibit
|
|
|
Muscle spindles Two outputs + One input
|
OUTPUTS
1a - coil around all the chain and bag fibres (fire when stretched) 2 - coil around chain/static bag fibres (fire even during static INPUT Efferent gamma motor neurons - shorten with contracting antagonistic muscle - allows max sensitivity (but then CNS needs to know that this shortening is occuring, not just due to muscle stretching) |
|
|
Tendon Organs vs Muscle Spindles
When they fire |
Tendon organs Fires when muscle contracts
Muscle spindles Fire when muscle is stretched = Parallel processing system |
|
|
Tendon organs' circuits + necessity of the muscle spindles
|
Can either act through 1b inhibitory interneuron - activate antagonistic, inhibit homonymous -> terminate
Locomotion - switch to excitatory -> activates homonymous, inhibits antagonistic -> spring in step = positive feedback (so regulated by spindle) |
|
|
Muscle Spindle's stretch reflex
|
Alpha output stimulates muscle -> streches extrafusal fibres + gamma output stimulates spindle -> stretches intrafusal spindle
When they don't match -> feedback adjusts the muscle output so output is exactly what you want e.g. in load compensation. |
|
|
Flexibility in muscle proprioception + effect of active people
|
Spindle gain can be adjusted (e.g. more sensitive if slow movements
Tendon Reflex can be reversed +less active people have lower relative stretch reflex strength except ballerinas because need to tame reflexes |
|
|
Fast vs Slow descending pathways
|
Fast pathways control specific movements
Slow pathways use amines/neuropeptides to modulate (metabotropic) BUT colocalised fast amino acid + biogenic amines + neuropeptides (with indepedent release |
|
|
Shik's midbrain experiment
|
Showed that stimulation intensity proportional to gait speed in cats
|
|
|
Raphe neurons in Locomotion
|
5HT neurons tonic firing (no movement) -> high frequency bursts (locomotion)
|
|
|
Which neuroamines slow/speed up CPG
|
5HT slows, Substance P speeds up
|
|
|
Treatment for descending spinal injuries
|
mimick glutamate/nmda release e.g. clonidine (if added soon after)
|
|
|
Cerebellar functions (x2) + how we demonstrate them
|
Coordinates movement (comparing actual vs planned - automating corrections in movement) shown by cooling - limb position oscillates
Learning - if lesioned - no new learning, Makes learned moves unconscious. PLUS not exclusively motor. |
|
|
Lateral cerebrocerebellar function inputs/outputs
|
Planning and initiation of movement and precision
sensory, motor, premotor, parietal input motor/premotor output |
|
|
Folia in cerebellum
|
In each lobe - lobules (folia) = basic conserved processing unit with massive parallel processing
|
|
|
Cortex of Folia (cerebellum) layers, inputs, outputs,
|
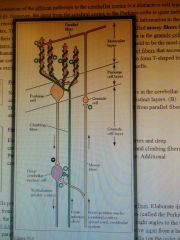
Molecular, Cellular, Granule cell layers.
Inputs from Mossy/Climbing fibres. Project onto Deep cerebellar nuclei |
|
|
Basics of cerebellar circuit + learning
|
Mossy fibres project to granule cell which has its own circuits with golgi cells, then projects to Purkinje (either directly or via stellate)
Climbing fibres project directly to Purkinje Cells (weakens granule output by LTD to Purkinje if they fire together). Purkinje cell is inhibitory to deep cerebellar nuclei-> counters excitatory output from direct MF/CF So if both climbing and granule fibres fire together -> LTD -> reduces Granule output to purkinje -> less inhibitory purkinje -> more cerebellar output |
|
|
Basal Ganglia function
|
Filter for motor commands
|
|
|
Basal Ganglia nuclei x5
|
Putamen, Caudate -> (striatum) Globus Pallidus, Subthalamic Nucleus, Substantia nigra
|
|
|
Direct and indirect circuits in basal ganglia
|
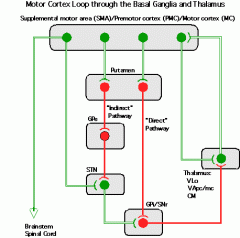
Direct=activating (disinhibits thalamus), Indirect= Repressive (encourages inhibition of thalamus)
|
|
|
Effect of Dopamine on Basal Ganglia
|
D1 receptors in striatum (activating), D2 in GPe (repressing) -> more direct pathway
|
|
|
Lesions to basal ganglia
|
Lateral -> always learning to walk
Ventral/Frontal -> addictions |
|
|
Huntingtons symptoms, cause
|
Uncontrollable movements, dementia, death + cognitive effects
Nerve cell death in striatum so tonic repression of indirect path. Because of >40 CAG repeats in huntingtons. |
|
|
Cerebellar Calibration of Oculomotor Reflex
|
Input into flocculus.
Climbing fibre input mediates plasticity at parallel fibre:purkinje cell synapse - if less excitation of purkinje -> more output of cerebellum -> changes VOR. Although more complex, because often more firing of purkinje -> more VOR |
|
|
How do we decide what to look at (two structures)
|
Retina/Auditory/Tactile projections onto superior colliculus - basal ganglia tonically inhibits, until cortex makes decision, then disinhibits.
|
|
|
Hebbian Plasticity
|
If Cell A fires before Cell B - their connection should be strengthened. i.e. fire together, wire together
|
|
|
Mechanisms of Presynaptic plasticity x5
|
Homosynaptic Facilitation = more NT released because more calcium sequestered
Homosynaptic Depression = habituation learning because low NT, eventual inactivation of Cav Short-term Heterosynaptic facilitation = sensitization to strong stimulus by facilitator interneurons activating (serotinergically) presynaptic GPCRs -> more NT/lowering threshold Short-term heterosynaptic dishabituation - lifts habituation via interneurons sertoninergically activating PLC GPCRs (similar to facilitation) Long term heterosynaptic facilitation - PKA translocates to nucleus, phosphorylates CPEB -> more active zones + prolonged PKA activation |
|
|
Postsynaptic contribution to presynaptic facilitation
|
Calcium Chelator BAPTA postsynaptically blocks it
|
|
|
Hippocampal LTP
|
Single shock to inputs (perforant paths, schaffer collaterals) -> evoked potential that can be recorded extracellularly because all dendrites same way -> shows long term LTP
|
|
|
Three factors of LTP
|
Input specificity - Doesn't affect other synapses
Cooperativity - Need both pre and post to interact Associativity - can affect other synapses if input paired |
|
|
Mechanism of LTP and how to stop it
|
Glutamate binds to postsynaptic AMPA -> depolarises -> releases Mg2+ block in NMDA -> Ca2+ in through NMDA -> CamKII -> phosphorylates existing AMPA + inserts new ones
If Mg2+ added - stops it + if calcium chelated -> no LTP so must be rise in Calcium. Protein synthesis blocker stops long lasting (because no new AMPA) |
|
|
Induction of LTD (two ways)
|
Either 1s-1 input -> slow rise of calcium -> favours phosphatase, rather than kinase
Or LTP induces it in adjacent synapsesi |
|
|
Synaptic Tagging in LTP
|
If protein synthesis blocked in one temporarily (while both tetanised -> LTP) -> both can be LTP, because tetanising tags synapse.
|
|
|
Uses of Plasticity x6
|
Phase change in locus (applying 5KT)
NMJ (competition where active fibres reinforced, others inactivated) Cortices development (e.g. somatosensory, V1) Spatial Learning (e.g. in hippocampus - NMDA blocked- > impaired) Cerebellum (weighting of parallel:purkinje synapse) Learning e.g. conditioned reflexes (interpositus) |
|
|
Metaplasticity
|
Once LTP/LTD have occurred, the threshold for LTP/LTD changes e.g. if depressed, becomes much easier to activate e.g. in determining stripes in V1/LGN
|
|
|
Push - pull model of motivation
|
Pull from Goal + Push from internal homeostasis.
As opposed to drive - only push. |
|
|
Why is the hypothalamus a likely drive centre (input/outputs)
|
Inputs from sensory centres, cortices, access to blood, temp+osmo -> see all the needs of body + wants
Outputs -> Neuroendocrine (pituitary) + Autonomic (brainstem) + cortex (behavioural) |
|
|
Lateral Hypothalamus drive centre theory + problem
|
if lesioned electrolytically -> aphagia
Stim elec or chem (NA) -> induces eating Electrophysiology -> sensitive to food if hungry BUT not necessary and sufficient - damage to mfb also when lesioning |
|
|
Excitotoxic lesioning of LH
|
DA Excitotoxic lesions to LH -> deficit in consumption of motivated behaviour
Glutamatergic excitotoxic lesions to LH -> deficit in ingestive i.e. consummatory(can recover partially) |
|
|
Dual Centre hypothesis for Feeding
|
LH = hunger centre, VMH = satiety
WRONG |
|
|
Factors for meal initiation (x3)
|
Environmental - sight/smell/taste
Internal - gluco/lipoprivation Ghrelin - stomach only secretes when empty |
|
|
Signals to stopping hunger (x4)
|
Gastric distension
CCK from duodenum Taste/smell/swallow food Long term - leptin released from well nourished adipose, reduces sensitivity to ghrelin etc. in Arcuate |
|
|
Neurocircuits of feeding (x3)
|
Arcuate Nucleus (affected by ghrelin/leptin) produces Neuropeptide Y, Agouti-Related Peptide => project to orexin/MCH neurons in Lateral Hypothalamus.
(If NPY/AGRP injected -> voracious eating) +Arcuate's CART/alpha-MSH -> LH -> inhibition +Arcuate -> paraventricular -> metabolic rate/insulin |
|
|
Detection of Thirst (x4) and reaction by MPN
|
Osmometric = interstitial fluid becomes hypertonic -> AV3V/OVLT
Volumetric thirst = when less fluid all together -> kidney releases renin -> Angiotensinin to AII ->stimulates SFO Atrial Baroreceptors -> AV3V Osmoreceptors in stomach All converge on median preoptic nucleus -> stimulate vasopressin and controls drinking |
|
|
Sexual Behaviour Consumption
|
Hormone dependent (androgen receptors in mPOA in males, oestradiol receptors in VMH of females).
Still shows preparatory/appetitive behaviour i.e. hormones affect consumption |
|
|
Dopaminergic lesions to mfb/striatum
|
Similar Aphagia/Adipsia responses (aka LH syndrome)
Nigrostriatal dopamine system allows activation of expression of motor i.e. consummatory. |
|
|
Mesolimbic Dopamine pathway
|
Medial Midbrain VTA to Ventral striatum (not dorsal as in motor). Involved in motivation + rewards + reward learning
|
|
|
ICSS
|
Intercranial Self Stimulation - self administer current to mesolimbic DA pathway -> innervating nucleus accumbens
|
|
|
Nucleus accumbens
|
Responsible for reward system, and without it - won't show any preparatory/appetitive behaviour i.e. opposite to hypothalamus.
Dopamine released into Nucleus accumbens in presence of goal/CS (i.e. peak of dopamine shifts to CS after initial firing) i.e. reward correct predictions -> supports reinforcement instrumental learning. |
|
|
What does Phineas Gage tell us about emotion
|
Tamping iron through frontal -> changes emotions
|
|
|
What does Kluver Bucy tell us about emotion
|
Temporal Lobe Lesions -> tameness + visual agnosia + hypersexuality i.e. disconnect between sensory and affective properties of stimulus (also because of amygdala damge)
|
|
|
Basolateral Amygdala inputs/outputs/function
|
Cortical section that gets sensory inputs (processed and unprocessed), projects to hypothalamus, frontal lobes, brain stems, thalamus, nucleus accumbens (so everything)
Involved in assessment of emotional significance of stimuli. |
|
|
Lesioning BLA
|
Blocks Pavlovian conditioning - site of learning for fear response i.e. different to reward based associative learning. BUT also affects appetitive responses to light (means copulation in rats)
Plus activated w/ emotional faces. |
|
|
Amygdala/NA vs Hypothalamus/mfb
|
Appetitive vs Consummatory
|
|
|
Effect of Cocaine on BLA/NA functions
|
increases Dopamine, so increases the salience and importance of drug related stimuli -> more drug response
|
|
|
Visual Agnosia
|
Damage to what pathway -> not recognising stuff
|
|
|
Ventral Stream vision
|
V1 -> V2 -> V4 (colour) -> Inferotemporal (complex e.g. faces)
|
|
|
Grandmother Cells
|
Specific cells for each individual object unlikely - because cell death - no recognition, therefore combination of different things
|
|
|
Dorsal Stream vision
|
V1 -> V2 -> V5/MT (direction) -> MST (motion - akinetopsia) -> parietal
|
|
|
Ventral vs Dorsal in Matching to Sample Tasks
|
Either Landmark i.e. have to recognise where well is
Or Object Discrimination i.e. have to recognise one object compared to another Expected effect of lesions |
|
|
Cherry's Dichotic Listening
|
Shadow - only follows one
|
|
|
Treisman Dichotic Listening
|
Showed track can switch without noticing -> filter
|
|
|
Posner Task
|
Exogenous / endogenous cues
|
|
|
RT in conjunction vs feature search
|
Because FIT i.e. serial spotlight search of space vs parallel search of feature modules
|
|
|
Synchrony in firing
|
Mechanism for binding objects together + activation -> neurons together (Hebb)
|
|
|
Dorsolateral Prefrontal Cortex vs Ventromedial/Orbitonfrontal Cortex
|
Dorsolateral Prefrontal Cortex ->
Inhibitory Control (Wisconsin card sorting) Working memory (Monkey remembering location) -> specific to lesion locations. Ventromedial Cortex Emotion (solicitation/submissive females in mating -> indiff/aggression) Stimulus Reward (won't extinguish learning) Somatic Marker Hypothesis (Iowa Gambling Task) |
|
|
Broca's and Wernicke's areas
|
L frontal lobe - broca's aphasia (speech production)
Posterior superior temporal gyrus - comprehension issues |
|
|
Broca's Aphasia and causes
|
Word production difficulties = effortful, telegraphic, lack of function words, agrammatic + some grammatical, syntactic comprehension.
But lesions need to be a little wider than Broca's area -> to get broca's aphasia. Is it because of Articulation deficit, economy of effort, syntactic impairment, |
|
|
Wernicke's Aphasia and deficits
|
Meaningless speech with many semantic errors, and comprehension poor.
Again lesions need to be wide and deep (to white matter) Primary deficit in analysis of acoustic input OR semantic deficit? Poor performance on semantic tests e.g. word-picture matching, but implicit tests like priming -> some semantic knowledge remains |
|
|
Problems in Lesion-Behaviour correlations
|
Functional reorg may follow brain damage
Individual variability Lesions vary across neuroanatomical/functional boundaries |
|
|
Spoken Language processing from brain imaging
|
Distributed representation - starting with auditory analysis in Heschl's gyrus, finer analysis along superior temporal gyrus.
HIckok/Poeppel's model shows dorsal and ventral bilateral temporal system |
|
|
Hemispheric language specialisation
|
Greater activation of LH in spoken language
Written Language primarily engages LH Marslen Wilson suggested syntax on LH, semantics bilateral. Some think Broca's for syntax, others think distributed. Experiments show that LIFG and LpMTG part of network, if either damaged -> impairs whole network. |
|
|
How we use semantic impairment to predict neural representations of semantics
|
Damage leads to gradual loss - so must be network not atoms
Damage affects some categories/types of features more than others eg. living things deficits with anteromedial temporal cortex lesions, so systems different for different categories? |
|
|
Category Specific Deficits model
|
May be due to evolutionary adaptation? supported by conditioning with snakes over flowers.
OR Feature based: Categories high in visual features rely on occipitotemporal regions, high in somatosensory rely on postcentral -> may explain category specificities. supported by some neuroimaging OR conceptual hierarchy - simple visual features first, then complex. So living things are quite similar, so need fine distinctions, but the distinguishing features of non-living things greater, so only needs coarse. also supported by neuroimaging + MEG shows anterior -> posterior processing (and interactive) |

