![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
A: analogous to blood smear, can examine individual cells and determine M:E ratio and presence of myeloid maturation
B: 3D section is fixed and sliced before being placed on a slide, can examine marrow architecture and overall cellularity |
Bone Marrow Aspirate vs Biopsy
|
|
|
Acute leukemias
|
Neoplastic cell: very immature, very early in the lineage.
Precipitous onset Effacement and replacement of the normal hematopoietic elements of the bone marrow result in pancytopenia and fatigue, bruising, bleeding, infections Infiltration of other tissue by the neoplastic cells may be responsible for some signs or symptoms. Without treatment, survival measured in days or weeks. |
|
|
Distinguish by clinical and microscopic findings, cytochemistry, cytogenetics, immunophenotype
A: Neoplastic cell shows features of commitment to the myeloid/ erythroid/ megakaryocytic/ monocyte lineage, Acute: subtypes classified by cytogenetics and morphology, Chronic: several related but distinct disorders. B: Neoplastic cell has features of commitment to lymphoid lineage, Acute: T vs B lineages; B has several subtypes, Chronic: usually B, but other types exist. |
Myeloid vs Lymphoid Leukemias
|
|
|
Chronic Leukemias
|
Insidious onset
Diagnosis may even be serendipitous Survival in the absence of treatment usually measured in years. Neoplastic cells show differentiation, and in some cases, ~function |
|
|
Chronic Myeloproliferative Disorders
|
Clonal disorders: transformed pluripotent stem cell or multipotent progenitor cell
One cell line gets a “proliferative advantage” but multiple lineages may derive from transformed cell. Progeny of transformed cell mature relatively normally. Hematopoiesis is effective, at least for a while |
|
|
Most common CMPDs
|
CML
Polycythemia rubra vera Primary myelofibrosis/Agnogenic Myeloid Metaplasia Essential Thrombocytosis |
|
|
CML
|
Clonal stem cell disorder
t(9;22) - BCR/ABL fusion protein has constituitive tyrosine kinase activity, deregulating signal transduction pathways - leads to abnormal cell cycling, inhibition of apoptosis, and increased proliferation of cells Characterized by 3 sequential phases: Chronic, Accelerated, Blast-crisis - each is progressively more resistant to therapy |
|
|
CML; bone marrow aspirate
|
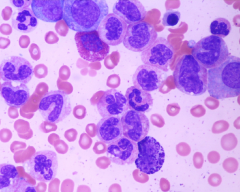
note all stages of the granulocyte family tree are present; during the stable phase, the neutrophilic myelocytes are the most numerous
|
|
|
CML; peripheral blood smear
|
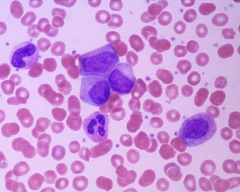
small number of blasts and promyelocytes seen; sometimes can look like reactive leukocytosis but other Dx tests (LAP, 15 etc) can help establish Dx
|
|
|
Leukocyte Alkaline Phosphatase
|
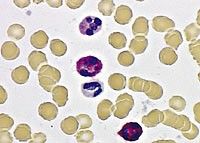
Enzyme found in mature neutrophils
Activity is increased in neutrophilias related to infection and inflammation Activity is increased during pregnancy Activity is LOW in CML Activity normal or increased in other CMPDs Red staining indicates LAP activity. Score 100 cells: 0, 1+, 2+, 3+ or 4+ based on intensity |
|
|
Imatinib
|
"designer" treatment for CML
Binds to ATP-binding site and inhibits BCR/ABL fusion protein activity |
|
|
Clinical course of CML
|
Chronic or stable phase: Relatively asymptomatic, +/- splenomegaly, fatigue, weight loss, bleeding
Accelerated phase: Loss of response to therapy, +/- fevers, bone pain, increased weight loss, increasing splenomegaly, increasing #s of blasts in marrow and blood. Blast crisis: 20% or more blasts in marrow and/or peripheral blood |
|
|
Blast crisis
|
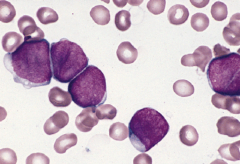
Blast crisis is defined as 20% or more blasts in the marrow or peripheral blood
|
|
|
Blast crisis
|
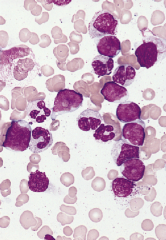
Blast crisis is defined as 20% or more blasts in the marrow or peripheral blood
|
|
|
A: Philadelphia chromosome
Decreased LAP score. Accelerated phase and blast crisis ~inevitable in absence of treatment B: Mutations of JAK2 frequent. LAP score normal or increased. Progression to AML varies and is NOT inevitable All can be complicated by hepatosplenomegaly and marrow fibrosis |
CML vs. other CMPDs
|
|
|
CMPDs
|
CML
Polycythemia vera Essential thrombocytosis Myelofibrosis (Primary, Post-P vera, Post-thrombocytosis) |
|
|
Essential thrombocytosis
|
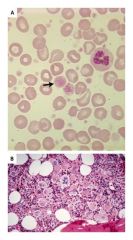
Increased platelets in blood
Increased megakaryocytes in bone marrow |
|
|
Idiopathic (primary) myelofibrosis
|
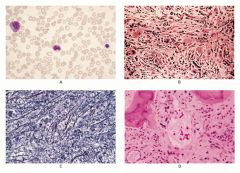
|
|
|
Extramedullary hematopoesis
|
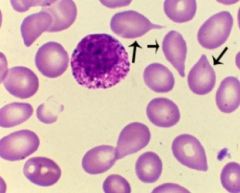
Production of blood cells outside the confines of the bone marrow
Most commonly spleen, but also liver, skin, lymph nodes Not seen in acute leukemias or lymphoproliferative disorders May develop in severe chronic hemolytic anemias, especially beta thalassemia major RBCs have "teardrop" morphology |
|
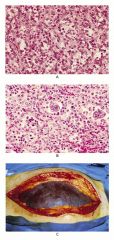
|
A: normal spleen
B: spleen with extramedullary hematopoesis from a Pt with idiopathic myelofibrosis C. Spleen from Pt in 'B' |
|
|
Polycythemia rubra vera
|
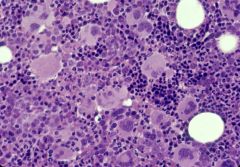
Erythropoietin level will be low in P vera. In patients with secondary polycythemias, erythropoietin level will be high.
Despite the erythroid lineage having the “proliferative advantage” in P vera, there are also abundant megakaryocytes evident in this bone marrow specimen |
|
|
Lab features of P vera, Essential thrombocytosis, and idiopathic myelofibrosis
|
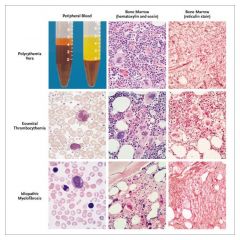
In P vera the increased Hct is remarkable.
In Essential Thrombocytosis, the platelet count is remarkable. In Idiopathic (Primary) Myelofibrosis, the marrow is characteristically replaced by fibrous tissue; extramedullary hematopoiesis occurs. Clues on peripheral smear include tear drop RBCs, nucleated RBCs and immature granulocytes. Remember, all of these (and CML) may be complicated by marrow fibrosis. But, it would appear that the marrow fibrosis occurs sooner and more predictably in Idiopathic Myelofibrosis |
|
|
95% of P vera have what mutation?
|
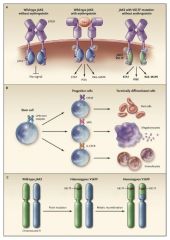
JAK 2 mutation - constituitively active, acts as though receptor has EPO bound
|
|
|
Acute Myeloid Leukemia
|
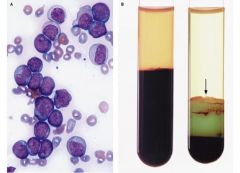
remarkable buffy coat; myeloid lineage - greenish color due to heme in MPO
|
|
|
Why perform leukophoresis pre-chemo treatment for AML?
|
reduce burden of neoplastic cells
|
|
|
What drug might you give when performing leukophoresis?
|
allopurinol
|
|
|
Why do you want to reduce the burden of neoplastic white cells in AML pre-chemo?
|
improved perfusion of small vessels, reduced debris from post-chemo "die-off" preventing DIC, less nucleic acid metabolites to form uric acid - no gout-related tissue damage
|
|
|
2 models of how AML clones develop
|
1. a previously normal hematopoetic stem cell acquires a sequence of mutations that alter how its growth is controlled as well as interfere with the ability of its progeny to differentiate
2. mutations arising in an at least partially differentiated cell restore gene-expression patterns that allow this cell to reacquire the unique self-renewal properties of stem cells while also interfereing with the ability of its progeny subsequently to differentiate |
|
|
AML
|
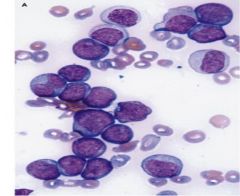
neoplastic blast cells appear homogenous and undifferentiated, actually they are a heterogenous collection that recapitulate the hierarchy of early precursor cells in blood cell differentiation
only a small subgroup retains the capacity to proliferate and self renew - leukemic stem cells - dependent on Wnt |
|
|
Wnt pathway signaling in AML
|
pathway inhibits destruction of beta-catenin
beta-catenin translocates to nucleus and complexes with TCF, promoting transcription of c-Myc and cyclin D1 |
|
|
AML risk factors
|
radiation
chemotherapy with alkylating agents (5q-, 7q-) chemotherapy with topo II inhibitors: MLL gene translocation benzene congenital conditions associated with genomic instability myelodysplastic syndrome or CMPD |
|
|
2 methods of classifying AMLs
|
FAB (french american british)
WHO |
|
|
FAB scheme for AML classification
|
8 categories: M0-M7
Based on degree of maturation and lineage Uses light microscopy, cytochemistry, immunophenotype, cytogenetics to classify |
|
|
WHO scheme for AML classification
|
special categories based on specific cytogenic findings that have particular prognostic importance
t(15;17), Inv16, t(8;21), t(11q23;v) |
|
|
WHO (broad) categories of AML
|
AMLs with well defined genetic aberrations
AMLs associated with myelodysplastic syndromes AMLs following chemotherapy AMLs not otherwise specified |
|
|
Auer rods
|
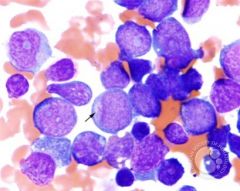
aberrant primary granules with MPO activity, not specific for a particular FAB M type or genetic finding, but indicate a NON-lymphoid leukemia and neoplastic process
|
|
|
FAB M0
|
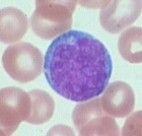
No single defining cytogenetic abnormality
Myeloperoxidase (MPO) negative, no Auer rods Expresses early myeloid antigens on immunophenotype Prognostic category: intermediate Remember: TdT negative |
|
|
FAB M1
|
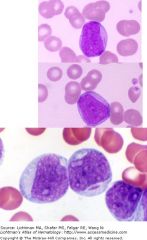
No single defining cytogenetic abnormality
Some cells MPO+ Some cells may contain Auer rods Prognostic category: Intermediate |
|
|
FAB M2
|
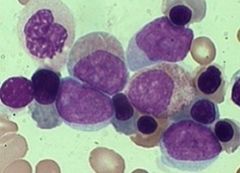
Commonest AML
At least two prognostic sub- categories: t8;21 positive associated with favorable prognosis. t8;21 negative: intermediate prognosis Blasts & some more mature cells: a few promyelocytes, myelocytes, maybe even later ones Blasts MPO + and may contain Auer rods |
|
|
FAB M3
|
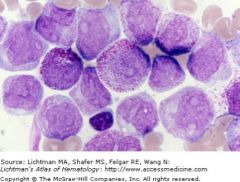
t15;17 defines the category
Predominant cell is the promyelocyte Neoplastic promyelocytes contain thromboplastin-like substance in granules: DIC is additional problem. Prognostic category: Intermediate |
|
|
FAB M3
|
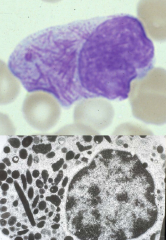
The t15;17 splits the retinoic acid receptor on 17 and inserts the PML gene from 15. A fusion protein results that suppresses retinoic acid receptor gene expression, thereby interfering with normal myeloid maturation.
Treatment includes administration of all-trans -retinoic acid |
|
|
FAB M4
|
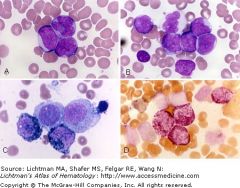
Neoplastic population: a mixture of immature cells with myeloid features (myloperoxidase (MPO) + and chloroacetate esterase +) and cells with monocytic features (non-specific esterase +).
Prognostically heterogeneous Includes favorable, intermediate and poor sub-categories. inv16 often found Panel C shows a cytochemical stain for MPO activity (black granules); two blasts are positive, two negative. Panel D shows a double esterase stain where myeloid cells stain red and monocytic cells are black. |

