![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Define Physical Property |

Can be observed or measured without changing the identity of the matter. |
|
|
What is Density? |
How solid/ heavier the matter is related to something else. |
|
|
The formula for density... |

|
|
|
What is mass? |
The amount of "matter", "weight" an object has on earth and in the cosmos. |
|
|
Define solubility |
The ability of a substance to dissolve in another substance. |
|
|
Name the 5 States of Matter |
1) BE Condensate |
|
|
What is Plasma? |

The cells are extremely hot and spread out to create extreme light. |
|
|
What is Bose-Einstein Condensate? |
The cells are extremely cold and clump together. |
|
|
What is a chemical property? |
• It describes matter based on its ability to change into new matter with different properties. |
|
|
Define flammability. |

• ability to burn |
|
|
What does nonflammable mean? |

NOT having the ability to burn |
|
|
Why do you use a Triple Beam Balance? |

Tool for measuring mass. |
|
|
Define volume |
The amount of space something takes up. |
|
|
What is a graduated cylinder? |

Tool for measuring volume |
|
|
What are grams? |
|
|
|
Independent variable |
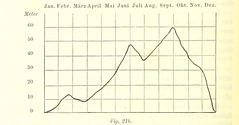
The variable controlled by the scientist. Data located on the x axis |
|
|
Define Dependent variable. |
Data located on the y axis. |
|
|
What is the relationship between mass and volume? |
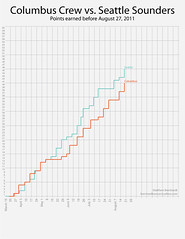
as mass increases the volume increases |
|
|
What is so special about Density of Gold 19.32 g/cm3? |

No matter what the size, gold will always have a density of 19.32g/cm3 |
|
|
What is important to remember when heating liquids? |

The student should point the sample away from everyone. |
|
|
What are goggles? |

Safety equipment worn when heating/ mixing, or working with chemicals and glassware. |
|
|
How should you mass Irregular shaped objects? |
Use water displacement method. |
|
|
What is the "Water Displacement Method"? |

The volume, (amount of water that rises), when an object is put into water. |
|
|
What should you do if glassware broke? |
Tell the teacher immediately. |
|
|
What is chemistry? |
The study of matter and its properties. |
|
|
What do geologists do? |
Study the various landforms on Earth. |
|
|
What is matter? |
Anything made up of mass and volume. |
|
|
Define a solution in chemistry. |
A mixture where the particles are evenly placed. |
|
|
What are elements? |
A pure item. |
|
|
define solids? |
Matter that can hold its own shape. |

