![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
Define the terms: atomic number, mass number and isotope. |
Atomic number: The number of protons in the atomic nuclei of a chemical element Mass number: The total number of protons and neutrons (i.e. the number of nucleons) in an atomic nucleus of an atom Isotope: Types of atoms that have the same number of protons but different number of neutrons in their nuclei |
|
|
Define the term of mole and give the value of Avogadro constant. |
1 mole is the amount of a chemical substance that contains as many elementary entities (atoms, molecules, ions, electrons or photons) as there are atoms in 12 g of carbon-12 isotope. 6.02×10^23 mol^−1 |
|
|
Define the term of electronegativity and the direction of its change within the rows and columns of the periodic table of elements. |
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons to itself. It increases to the right in the rows and upwards in the columns of the periodic table. |
|
|
Characterize the σ bond. (Sigma bond) |
The σ bond is the strongest covalent bond due to the fact that the bonding electrons can be found in a rotationally symmetrical arrangement along the axis of the bond. Single covalent bonds are typically σ bonds. |
|
|
Characterize the π bond. (pi bond) |
The second and/or third bond of multiple covalent bonds is a so called π -bond. The bonding electrons can be found above+under and/or behind+in front of the axis of the bond. It is weaker than the σ bond. |
|
|
Give the hybrid state of the carbon atom and the bond angles around the carbon atom in ethane, ethene (ethylene) and ethyne (acetylene) molecules. |
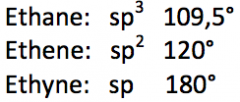
|
|
|
List the intermolecular chemical bonds in the order of increasing bond energy. |
1) van der Waals-interactions (hydrophobic interactions, London dispersion interactions) 2) Dipole-dipole interaction 3) Hydrogen bond |
|
|
Name the molecular criteria necessary for hydrogen bond formation. Name physical properties that may refer to the presence of hydrogen bonds. |
The donor of the H-bond must contain a polarized E-H bond (E=element, mostly N,O, F) whereas its acceptor must contain a free (nonbonding) electron pair. The presence of H-bonds can cause high melting point and boiling point (compared to the molecular weight) or good water solubility. |
|
|
How to calculate absolute temperature values (Kelvin scale) from or to the centigrade (Celsius scale)? Write an equation with explanation of the denotations. |
T = t + 273.15 where T = absolute or thermodynamic temperature(given in Kelvin) t = is the temperature in degree Celsius |
|
|
Write the equation of the ideal gas law with the explanation of the denotations. |
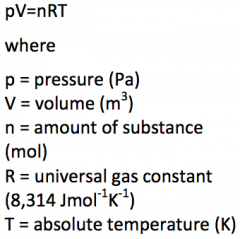
|
|
|
Define the terms: mass percent, mass-per-volume percent and molarity |
Mass percent: x g solute / 100 g solution Mass per volume percent: x g solute / 100 mL solution Molarity: x mol solute / 1 L solution |
|
|
Define the term: colligative property. Name four colligative properties. |
Colligative properties are properties of substances that are assumed to depend on the ratio of the number of solute particles to the number of solvent molecules in a solution, and not on the type of chemical species present. Examples: - lowering of vapour pressure - elevation of boiling point - depression of freezing point - osmotic pressure |
|
|
Write the equation of the osmotic pressure law with the explanation of the denotations. |
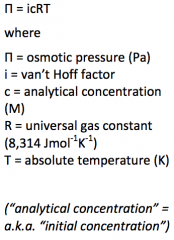
|
|
|
Define the terms: isotonic, hypotonic and hypertonic solution. Give the percentage andmolarity of the physiological saline. |
In isotonic solutions the osmotic pressure is equal to the intracellular osmotic pressure. Πsolution = Πcell In a hypotonic solution: Πsolution < Πcell In a hypertonic solution: Πsolution > Πcell The concentration of the physiological saline: 0.9 % (= 9 g/L) or 154 mM NaCl |
|
|
Describe briefly the molecular level events taking place during hemodialysis. |
The blood of the patient is driven through a tube surrounded by a semipermeable dialyzing membrane, which allows the small molecules pass through but withholds the colloidal sized ones (like proteins, colloidal lipids, cells). The blood is dialysed against a dialyzing liquid,which is isotonic to blood and contains essential molecules (e.g. glucose) in the same concentration as blood. |
|
|
Classify the colloids by the states of the phases. |
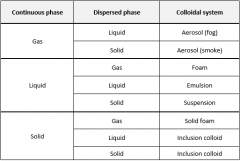
|
|
|
Define the terms: strong and weak electrolytes and non-electrolytes. |
Electrolytes: Substances which dissolve in water to produce conducting solutions of ions (e.g. NaCl) Non-electrolytes: Substances which neither dissociate nor produce ions in aqueous solutions. (e.g.sucrose) Strong electrolytes dissociate completely in aqueous solutions, α ≈ 1 Weak electrolytes dissociate only partially, α << 1. |
|
|
Give the definitions of acids and bases by Brønsted-Lowry and also by Lewis. |
Brønsted-Lowry: Acids are proton donor molecules or ions. Bases are proton acceptor particles. Lewis: Acids are electron pair acceptor molecules or ions. Bases are electron pair donors. |
|
|
Give the definition and possible range of the term: degree of dissociation. Write the definition of the acid dissociation constant of a weak acid (HA). |
Degree of dissociation is the ratio of the number of the dissociated particles and the total number of particles. 0 ≤ α ≤ 1 Ka = [H+][A-]/[HA] |
|
|
Define the chemical equilibrium constant for a general equilibrium system. Write an equation (mass action law) and explain the denotations. |

|
|
|
What does the Le Chatelier-Braun principle state? |
When a system in equilibrium experiences a stress (e.g. change in the concentration, temperature,or in case of gases pressure), a new equilibrium is settled that relieves stress. |
|
|
Give the value of the water ion product at 25°C! Define the terms pH and pOH and give their relationship. |
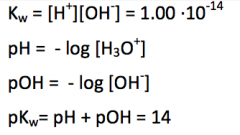
|
|
|
What do buffer solutions consist of? Define the terms: buffer effect and buffer capacity. |
Composition of the buffer solutions: A solution of a weak acid and its conjugate base (acidic buffer), or a solution of a weak base and its conjugate acid (alkaline buffer). Buffer effect: Diminishes changes in pH when acid or base is added. Buffer capacity: The amount of a monoprotic, strong acid (e.g.HCl) or monobasic, strong base (e.g. NaOH) in moles that should be added to one litre of buffer in order to change the pH of the bufferby one unit. |
|
|
Write the Henderson-Hasselbach equation and explain the denotations! |

|
|
|
Characterise the chemical bonds that hold the metal ions and the ligands together in metal complexes. |
In the complexes a special covalent bond, the so called dative (coordinative) bond is present. In this bond both bonding electrons are donated by the ligand and accepted by the metal ion. If one ligand donates more than one bonding pair to the same metal ion, than a quite stable, so called chelate complex is formed. |
|
|
Define the terms: exothermic, endothermic, exergonic and endergonic processes. |
In an exothermic process the enthalpy of the system decreases ( the enthalpy of the products is smaller than the enthalpy of the reactants), ΔH < 0 the energy is released in the form of heat, whereas the enthalpy of anendothermic process increase, ΔH > 0. if ΔG < 0 the reaction happens spontaneously, it is exergonic if ΔG > 0 the reaction does not go spontaneously, it is endergonic |
|
|
Write the Gibbs equation with the explanation of the denotations. Give the relationship between the direction of the processes and the sign of the free energy change. |

|
|
|
Depict the transition state and the activation energy of a reaction in a graph. What is thedefinition of a catalyst? |
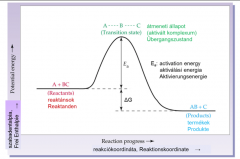
The catalyst opens a new reaction route with a lower activation energy and thus accelerates the reaction. |
|
|
Define the terms: oxidation, reduction, oxidizing agent, reducing agent. |
oxidation: loss of electrons reduction: electron uptake oxidizing agent: takes electrons from other substances reducing agent: gives electrons to other substances |
|
|
Write the Nernst equation of the electrode potential with the explanation of the denotations. |

|
|
|
Draw a galvanic/voltaic cell, write its cell diagram and define its electromotive force. |
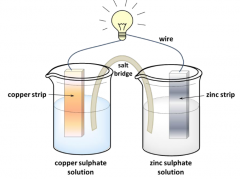
(-) Zn(s)|Zn2+||Cu2+ |Cu(s) (+) Eel = εk-εa Eel : electromotive force εk and εa : electrode potentials of the cathode and the anode, respectively. |
|
|
Draw with structures three molecules (not ATP) containing high energy phosphate bonds.Circle the high energy phosphate bonds. |
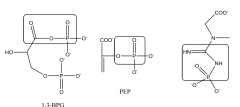
1) 1,3-bisphosphioglycerate 2) phosphoenolpyruvate 3) creatine phosphate |
|
|
Describe briefly the molecular explanation of the toxicity of cyanide ions and carbonmonoxide, respectively. |
Carbon monoxide binds strongly to hemoglobin and prevents the binding of oxygen. Cyanide bind to the heme in cytochrome oxidase (Complex IV) and inhibits its normal function |
|
|
Give the essential components and the osmolarity of the Ringer’s solution. |
Na+, K+, Ca2+, Cl- 305-315 mOsm Advanced versions may contain acetate, lactate, bicarbonate. |
|
|
Define the term: constitutional isomerism. Give examples! |
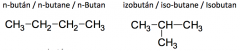
The constitutional isomers differ in the connection order of their atoms. |
|
|
Define the term: configurational isomerism. Give examples! |
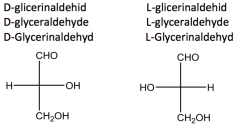
The configurational isomers differ in the relative steric position of the substituents. |
|
|
Define the term: conformational isomerism. Give examples! |
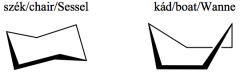
The conformational isomers form as a result of the rotation around sigmabond(s). |
|
|
Define the terms: enantiomers, diastereomers. Give chiral examples. |
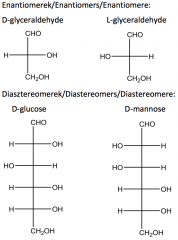
Two non-super imposable stereoisomers that are mirror images of each other are called enantiomers. Stereoisomers which are not mirror images of each other are called diastereomers. |
|
|
Draw with structure a primary alcohol, a secondary alcohol, a tertiary alcohol, an enol and a phenol. |
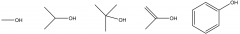
|
|
|
Name the products of the oxidation of aldehydes (1), the reduction of aldehydes (2) and the reduction of ketones (3), respectively. Name the products of the reactions of aldehydes with primary amines (4), alcohols (5) and aldehydes (6), respectively. |
1. carboxylic acid 2. primary alcohol 3. secondary alcohol 4. Schiff’s base 5. hemiacetal 6. aldol |
|
|
Draw with structures a primary amine, a secondary amine, a tertiary amine, a quaternaryammonium salt. Give the order of base strengths of the amines in aqueous solution. |
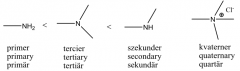
|
|
|
Draw with structures cholin, colamine and acetyl choline. |
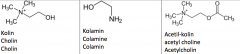
|
|
|
Draw with structures urea, guanidine and creatinine. |
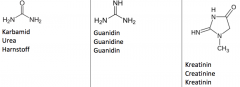
|
|
|
Draw with structures pyrrole and imidazole. Characterize their acid-base properties. |

|
|
|
Draw with structures pyridine, pyrimidine, indole and purine. Give the numbers of theatoms also. |
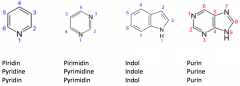
|
|
|
Define the term: alkaloid. |
Alkaloids are a group of naturally occurring compounds that contain mostly basic nitrogen atoms and possess physiological and/or pharmacological activities. |
|
|
Draw the structure of the reduced glutathione. Circle the part of the molecule responsible for its antioxidant property. |
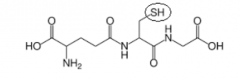
|
|
|
Give the definition and two most frequently used units of enzymatic activity. |
The activity of an enzyme equals to the amount of substrate transformed by the enzyme within a unit time at 25°C. Units: - Unit (U): μmol/min - katal: mol/s |
|
|
Write the Michaelis-Menten equation with the explanation of the denotations. |

|
|
|
Draw the structural formula of cholesterol, Give the numbers of the atoms also. |
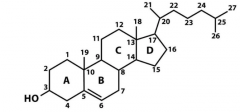
|

