![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
100 Cards in this Set
- Front
- Back
|
List and define all cardiomyopathies |
1. Dilated cardiomyopathy: eccentric LV hypertrophy causing impaired systolic function
2. Hypertrophic cardiomyopathy: concentric LV hypertrophy causing abnormal diastolic relaxation
3. Restrictive cardiomyopathy: LV noncompliance with total dysfunction, diastolic dysfunction > systolic dysfunction. This is typically related to fibrosis or deposition of an abnormal substance in the LV.
4. Inflammatory cardiomyopathies: myocyte necrosis/damage mediated by inflammatory process
5. Misc cardiomyopathies: - Arrhythmogenic RV dysplasia, a fatty infiltration and fibrosis of the RV - Isolated LV noncompaction: prominence of trabeculated tissue - Stress cardiomyopathy: apical ballooning as a consequence of catecholamine flood - Ion channelopathies |
|
|
Pathologic evidence for stress cardiomyopathy |
Contraction bands |
|
|
Etiologies of diated cardiomyopathy |
1. Idiopathic (most) 2. Inflammatory (infectious, immune) 3. Toxic: alcohol, cocaine, chemo (dose-dependent, reversible) 4. Pregnancy-associated (peripartum cardiomyopathy) 5. Genetic: muscular or myotonic dystrophy |
|
|
Structural abnormalities present in dilated cardiomyopathy (4) |
1. Dilation and thickening of LV (but dilation is vastly out of proportion to LV --> walls appear thin).
2. LA, RA and RV dilation, but not as prominent as LV dilation
3. Widening of mitral annulus (may cause mitral regurgitation if severe enough) |
|
|
Pathophysiology (and compensatory pathophysiology) of dilated cardiomyopathy |
1. Impaired systolic function and cardiac output (hemodynamic hallmark) 2. Compensatory increase in inotropy 3. Compensatory increase in preload 4. Decompensation (heart failure outpaces compensatory mechanisms). |
|
|
Physical exam: dilated vs hypertrophic cardiomyopathy |
Dilated cardiomyopathy: S3 gallop w/laterally displaced apical impulse
Hypertrophic cardiomyopathy: S4 gallop |
|
|
Dilated cardiomyopathy: clincal picture |
1. Low cardiac output 2. Congestive heart failure 3. Compensatory mechanisms (i.e. tachycardia, vasoconstriction w/cool extremities) 4. Atrial dilation, afib and thromboembolism 5. Mitral regurgitation due to annulus expansion 6. Pulsus alternans (late-stage) |
|
|
Complications of cardiomyopathy (5) |
1. Thromboembolism from afib (strongest risk for dilated cardiomyopathy) 2. Ventricular arrhythmia, sudden death (more common in hypertrophic cardiomyopathy)
3. Infective endocarditis
4. LVOT from HCM: thrombus formation from contact of anterior mitral valve leaflet with LV wall
5. Dilated cardiomyopathy: LBBB --> dyssynchrinous ventricular contraction |
|
|
Treatment of dilated cardiomyopathy |
Medical 1. Reduction of preload - Diuretics - ACEi's (also vasodilate) - ARB or hydralazine/nitrate as ACEi alternatives 2. Inotropes for acute hypotension
Surgical 1. Pacemaker to reduce the risk of arrhythmias 2. Biventricular pacemaker for LBBB to induce synchronous ventricular contraction |
|
|
Etiology of hypertrophic cardiomyopathy |
Always genetic mutation of sarcomere (contractile apparatus) proteins |
|
|
Histological classification of cardiomyopathy |
1. Hypertrophic cardiomyopathy: nonspecific findings
2. Dilated cardiomyopathy: histology is diagnostic; architectual disorganization of myocytes w/lateral attachments
3. Restrictive cardiomyopathy: diagnostic for certain etiologies - Amyloid inclusions - Iron deposition - Pompe's disease (glycogen inclusions)
4. Inflammatory cardiomyopathy: inflammatory infiltrates - Neutrophils: indicative of bacterial process - Eosinophils: Loeffler's disease - Giant cells: TB/fungal/sarcoid - Lymphocytes: viral process |
|
|
Structural abnormalities in hypertrophic cardiomyopathy |
1. Small, banana-shaped, slit-like LV chamber; LV hypertrophy, predominantly in the interventricular septum
2. Intermittent LV outflow obstruction in 1/3 of cases (anterior mitral valve leaflet imposes on aortic outflow tract during systole)
3. May exhibit mitral regurgitation with severe LV outflow obstruction |
|
|
Hemodynamics: hypertorphic vs. dilated |
Hypertrophic cardiomyopathy: normal/high ejection fraction
Dilated cardiomyopathy: low ejection fraction |
|
|
Pathophysiology of LV outflow obstruction in hypertrophic cardiomyopathy |
Narrowed area of aortic valve → Venturi effect → decreased pressure through outflow tract → pressure vacuum → anterior mitral valve leaflet superimposition over outflow tract. |
|
|
Clinical picture: hypertrophic cardiomyopathy |
1. Angina & dyspnea in the absence of ischemic heart disease
2. Syncope w/exercise in cases with LV outflow obstruction
3. Sudden cardiac death |
|
|
Pathophysiology of angina & dyspnea in hypertrophic cardiomyopathy |
1. Hypertrophy --> increased myocardial oxygen demand 2. Reduced compliance --> reduced diastolic filling --> reduced coronary perfusion |
|
|
Treatment of hypertrophic cardiomyopathy |
1. Medical - Decrease myocardial oxygen demand: beta blockers and Ca blockers that reduce HR - Anti-arrhythmics - Antibiotic prophylaxis for endocarditis
2. Surgical/interventional - Myectomy - Pacemaker/defibrillator to reduce arrhythmia risk - Percutaneous alcohol septal ablation
|
|
|
Etiology of restrictive cardiomyopathies |
1. Primary - Endomyocardial fibrosis (African children & young adults) - Endocardial fibroelastosis (fatal early in life)
2. Secondary - Radiation fibrosis - Amyloidosis - Sarcoidosis - Hemochromatosis - Metastatic disease - Inborn errors of metabolism (i.e. Pompe's disease, an abnormality in glycogen storage) |
|
|
Structural changes in restrictive cardiomyopathy |
Depends on specific process. - Normal LV thickness and chamber size - Myocardium may be firm/opaque/abnormal in color
|
|
|
Diagnosis of restrictive cardiomyopathy |
- “Square-root sign” on EKG
- Important to distinguish from constrictive pericarditis (cardiac imaging) |
|
|
Treatment of restrictive cardiomyopathy |
1. Treat underlying cause - Amyloid: chemo/prednisone/colchicine - Hemochormatosis: chelation/phlebotomy - Hemochromatosis/endomyocardial fibrosis: steroids
2. Congestive symptoms: diuretics (but keep in mind they will decrease cardiac output)
3. Digoxin (inotrope, afib)
4. Antiarrhythmics for afib
5. Anticoagulation for thrombus formation (esp. in atria appendage)
6. Pacemaker placement |
|
|
Most common infectious processes implicated in inflammatory cardiomyopathy |
1. Most often viral (Coxsackie V & HIV)
2. Bacterial: commonly lyme
3. Fungi: candida
4. Protozoa (i.e. T. cruzi)
5. Helminthes (trichinosis from undercooked pork) |
|
|
Autoimmune causes of inflammaotry cardiomyopathy |
1. Allergic reactions (drug hypersensitivity) 2. Post-streptococcal (Rheumatic fever) 3. Post-viral 4. Systemic immune disease (lupus) 5. Transplant rejection |
|
|
Structural changes in inflammatory cardiomyopathy |
Immune cell infiltrates; frequently leads to dilated cardiomyopathy |
|
|
Defining features of shock |
1. Hypotension (systolic BP <90 mm Hg) 2. Elevated lactate levels in blood 3. Evidence of organ dysfunction - Kidney: low urine output - CNS: altered mental status - Lungs: acute respiratory failure
NOTE: by definition, shock implies a "decompensated state"; if for example BP is preserved w/blood loss, the condition has not yet progressed to proper "shock." |
|
|
Oxygen extraction ratio: definition, normal parameters and implications in shock |
Definition: oxygen extraction ratio = Normal parameter: 25%
Implications for shock: to compensate for a decrease in oxygen delivery, the oxygen extraction ratio will increase to preserve oxygen uptake by tissues. Once maximal O2 extraction is reached, oxygen uptake will decrease and organs will switch to anaerobic metabolism; this is officially a "decompensated" state. |
|
|
Why do patients in shock have such high oxygen uptake requirements? |
Shock often induces a hypermetabolic state |
|
|
Classification of circulatory shock |
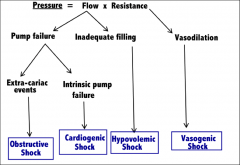
|
|
|
Etiology of each classification of shock |
Hypovolemic: blood loss
Cardiogenic: - Acute mitral regurgitation
Obstructuve: - Massive pulmonary embolism - Cardiac tamponade - Tension pneumothorax
Vasogenic shock: - Septic - Anaphylactic - Acute spinal cord injury - Adrenal crisis |
|
|
Is dehydration a version of hypovolemic shock? |
No; dehydration is a loss of asanguinous fluids. This entails a decrease in extracellular volume, but osmotic pressure draws fluid from the interstitium, and volume in the vascular compartment is preserved. |
|
|
Hemodynamic patters for each type of shock |
Hypovolemic: - Low end-diastolic pressure - Low SV & CO - High compensatory systemic vascular resistance
Cardiogenic/obstructive: - High end-diastolic pressure - Low SV & CO - High compensatory systemic vascular resistance
Vasogenic: - Low end-diastolic pressure - High cardiac output - Low vascular resistance |
|
|
Clinical progression of hypovolemic shock |
Stage 1 (<15% blood loss): Asymptomatic
Stage 2 (15-30% blood loss): Bp normal; limbs col; beginning of decline in urinary output
Stage 3 (30-45%): Hypotension, oliguria, altered mental status, high serum lactate; risk of mortality
Stage 4 (>45%): Death; hypotension that is refractory to volume resuscitation |
|
|
Clinical hallmark of cardiogenic and obstructive shock |
Pulmonary & peripheral congestion (only shock category w/high end-diastolic volume) |
|
|
Pathophysiology of vasogenic shock |
DYSOXIA!! Impaired mitochondrial (ETC) utilization of oxygen. Oxygen extraction is less than the normal 25% (unlike the other types of shock, where it is maximal) |
|
|
Resuscitation fluids |
1. Crystalloid fluids: isotonic saline; increases extracellular fluid volume
|
|
|
Early goals of shock resuscitation (3) |
• Mean arterial pressure >65 mm Hg (protect cerebral perfusion) • Urine output >-0.5 mL/kg/hr • Serum Lactate < 2 mmol/L within 24 hours |
|
|
Management of hypovolemic shock |
In order of urgency: 1. Volume resuscitation (colloids/crystalloids) to augment BP & urinary output
2. Hemoglobin resuscitation: packed RBCs
3. Hemostatic resuscitation: plasma and platelet concentrates to prevent coagulopathies |
|
|
Management of cardiogenic/obstructive shock |
1. Pharmacologic: positive inotropes w/alpha stimulation for BP (dopamine prerferred) 2. Intra-aortic balloon pump 3. Fix underlying condition |
|
|
Management of vasogenic shock |
1. Volume resuscitation w/colloid or crystalline fluid (capillaries get leaky when resistance is so low) 2. Vasopressor therapy (alpha-agonism) depending on kind of shock: - Septic: NE (beta to compensate for myocardial depression) + vasopressin - Spinal: phenylepherine (pure alpha-agonist) |
|
|
Embryologic origin of heart tissue |
Splanchnic lateral plate mesoderm (except for truncus ateriosus, which is neural crest) |
|
|
Cardiac embryology: Week 2 |
Cardiac precursors(first and second heart fields) form crescent and move to the midline (lateral folding) |
|
|
Cardiac embryology: Week 3 |
1. Angioblastic cords fuse to form a signle heart tube (the end of lateral foldoing)
2. BLood flow is established |
|
|
LIst the primitive heart structures from caudal to cranial end |
1. Sinus venosus 2. Primitve atrium 3. AV canal 4. Primitive ventricle 5. Bulbus cordis 6. Truncus ateriosus |
|
|
Cardiac embryology: Week 4 |
- Formation of endocardial cushions - Neural crest migration and contribution to conotruncus |
|
|
Week 5 events in cardiac embryology |
1. Cardiac looping (bulbus cordis)
3. Ventricular, atrial and aorticopolumonary septation |
|
|
Atrial septation |
1. Septum primum grows towards endocardial cushions
2. Septum segundum forms perforations in septum primum
3. Upper septum primumddegenerates
4. Remaining portion of septum primum forms valve of foramen ovale
5. Septum primum and segundum fuse to form atrial septum |
|
|
Ventricular & aorticopulmonary septation |
1. Muscular ventricular septum forms, with an interventricular foramen
2. Aorticopulmonary septum rotates ("spiral septum") and fuses with muscular ventricular septum to form membranous ventricular septum
3. Endocardial cushions grow and contribute to both muscular and membran |
|
|
Name the embryologic derivatives of each aspect of the heart |
Atrium: - Trabeculated atrium: primitive atrium - Smooth left atrium: pulmonary veins - Smooth right atrium: sinus venosuFetal circulation = high-resistance pulmonary vascular beds (right horn)
Ventricle: - Trabeculated ventricle: primitive ventricle - Smooth venricle: bulbus cordis (left & right)
Aorta & pulmonary veins: truncus arteriosus
Coronary sinus (right atrium): sinus venosus (left horn)
Superior vena cava: R common and R anterior cardinal veins |
|
|
Principal hemodynamic difference between adult and fetal circulation |
Fetal circulation = high-resistance pulmonary vascular bed
Adult circulation = low-resistance pulmonary vascular bed |
|
|
What closes the ductus arteriosus? |
Increase in oxygen --> decrease in prostaglandins --> closure of ductus arteriosus |
|
|
Clinical consequences of persistent pulmonary hypertension of the newborn, as well as management. |
Clinical consequences 2. Elevated R heart pressures and potential R heart failure 3. Patent PDA and PO 4. Cyanosis (Lower >>> upper extremities)
Management: sedation, iNO vasodilation and bypass (ECMO) |
|
|
Pathophysiology and compensatory mechanisms of L --> R shunts |
1. Decreased CO 2. R heart volume overload - RV hypertrophy - Pulmonary hypertension & effusion - Decreased pulmonary vasculature compliance
2. Compensatory mechanisms - tachycardia - Water retention - Catecholamine overload 9i.e. diaphoresis) |
|
|
Types of L --> R shunts |
1. Muscular VSD (inferior interventricular septum) 2. Membranous VSD (upper ventricular septum; due to error in fusion of septum primum/ septum segundum/ endocardial cushions) 3. ASD 4. Patent foramen ovale 5. Patent ductus arteriosus 6. AV canal defect: communication between all four channels (endocardial cushion defect) |
|
|
Diagnosis of patent ductus arteriosus |
L --> R shunt with cyanosis of lower extremities with crying (reverses shunt with increased pulmonary resistance) |
|
|
Eisenmenger syndrome |
Reversal of shunt as pulmonary resistance surpasses systemic vascular resistance |
|
|
List the R --> L shunts |
"The five T's":
1. Tetralogy of fallot 2. Transposition of the great vessels 3. Persistent truncus ateriosus 4. Tricuspid atresia 5. Total anomalous pulmonary vein return |
|
|
Defects in tetralogy of fallot |
1. Pulmonary stenosis 2. RV hypertrophy 3. Overriding aorta (covering VSD) 4.VSD |
|
|
Defect in transposition of the great arteries? |
Aorticopulmonary septum fails to take spiral course |
|
|
Defect in persistent truncus aterious |
Spiral septum fails to form |
|
|
Defect in tricuspid atresia |
Absence of tricuspid valve; hypoplastic RV |
|
|
Defect in total anomalous pulmonary vein return |
Pulmonary vein drains into R heart (associated with ASD/PDA to maintain CO) |
|
|
Surgical correction of single ventricle |
Stage 1: maintenance of PDA patency (prostaglandins)
Stage 2: separate pulmonary circulation (passively from RA) and systemic circulation (provided by contraction of normal ventricle).
Stage 3: Closure of all communication between venous and arterial blood; heart is entirely bypassed for venous return.
Why does it have to be staged? Pulmonary resistance must be low enough to support passive blood flow through pumlonary vasculature in the second stage, and the circulation must be adequately developed and prepared for stage 3. |
|
|
Consequences of coarctation of the aorta |
Aortic regurgitation |
|
|
Genetic syndromes contributing to congenital heart disease |
Trisomy 21: VSD, AV canal defect Trisomy 18: VSD, congenital polyvalvular disease Monosomy X: Aortic stenosis, coarctation of the aorta 22q11 (DiGeorge's): persistent truncus arteriosus, tetralogy of falot |
|
|
Classify lipoprotein particles by composition |
From most dense (primarily fat) to least dense (primarily cholesterol)
1. Chylomicrons 2. VLDL 3. VDL 4. HDL |
|
|
List and name the significance of each apo protein |
Apo B48: core protein for Chylomicron ApoB100: core protein for VLDL/VDL; ligand for LDL liver recpetor ApoE: extracts remnants from bloodstream (CM and LDLs after hydrolysis) ApoAI/ApoAII: HDL ineractions ApoCII: activates lipoprotein lysis |
|
|
Interaction between HDL and other lipoproteins |
Gives cholesterol to LDL (cholesterol ester transfer protein) |
|
|
What agents are crucial in the HDL uptake of cholesterol from peripheral tissue? |
Interaction with peripheral tissue: SR-B1 Conversion and storage of cholesterol: LCAT |
|
|
Interaction of insulin with lipoproteins |
Stimulates tissue lipoprotein lipase; inhibits hepatic lipoprotein lipase |
|
|
Lpa/Apoa? |
Makes LDL more atherogenic (biomarker for atherosclerotic disease). Mechanism is poorly understood. |
|
|
Interpretation of lipid panels |
LDL = TC - [HDL + TG/5] if TG <400 HDL is measured directly
Normal values: Total cholesterol < 200 |
|
|
Polygenic hypercholesterolemia |
Most common cause of elevated cholesterol
|
|
|
Familial hyperlipidemia |
Mutation in LDL receptor --> high circulating LDL and high lifetime risk for cardiovascular disease |
|
|
Familial combined hyperlipidemia |
Overproduction of VLDL, leading to high TG and TC.
Causes high serum LDL & VLDL and TG, as well as elevated lifetime risk for cardiovascular disease. |
|
|
Familial hypertriglyceridemia |
Overproduction of VLDL, leading to high TG but NOT high cholesterol.
Does NOT necessarily result in an incrased LLD/lifetime risk for cardiovascular disease |
|
|
ApoB100 mutation |
A much less common etiology of familial hypercholesterolemia, but with identical conseqeunces |
|
|
ApoE mutation |
"Remnant receptor disease:": High serum VLDL & chylomicron remnants, as well as high LDL (receptor competition)
|
|
|
Mutation in cholesterol ester transport protein |
Low LDL, high HDL and general protection from cardiovascular disease |
|
|
Lipoprotein lipase deficiency |
Elevated chylomicrons and seerely elevated cholesterol/TG |
|
|
SR-B1 mutation |
AKA Tangier's disease
Causes hypercholesteroleimia and low HDL; HDL never pick up cholesterol from tissues and are excreted by the kidneys
Manifestations: orange tonsils |
|
|
LCAT deficeincy |
Low HDL ("fish eye disease") |
|
|
Summary: what etiologies cause excess VDL? |
1. Too much synthesis - Eating it - Familial combined hyperlipidemia - Polygenic familial hypertriglyceridemia
2. LDL receptor deficiency - Downregulation from eating too much cholesterol - Familial hypercholesterolemia (LDL receptor mutation)
3. ApoE mutation (remnant receptor disease)
4. Defective ApoB100 |
|
|
Summary: what etiologies cause deficient HDL? |
1. Tangier's disease (SR-B1 mutation) 2. APoA1 deficiency 3. LCAT deficiency |
|
|
Dyslipidemia pathophysiology |
Two results: 1. Excessive cholesterol consumption --> downregulation of LDL receptor coupled with overproduction of VLDL
2. Obesity with insulin resistance --> - Increased HDL catabolism (less inhibitory effect on hepatic lipoprotein lipase - Decreased peripheral lipoprotein lipase activity --> more atherogenic LDL particles |
|
|
Types and etiologies of fluid in the pericardial space |
1. Serous (transudative) - CHF - Serum hypoproteinemia (i.e. w/liver failure) - Hypothyroidism
2. Sanguinous - Trauma (i.e. inappropriate catheter placemetn) - Ventricular rupture following lMI - Aortic dissection with rupture into the pericardial sac - Coagulation pathologies - Metastatic tumor - Tuberculosis
3. Fibrinous (w/excessive serous proteins; usually extravasated from vessels)
4. Purulent
5. Exogenous (i.e. TPN)
6. Chylous (lymphatic): surgical severance of the thoracic duct
7. Caseous (TB/fungal/sarcoidosis) |
|
|
Etiologies of air in the pericardial space |
1. Trauma - Positive pressure ventilation - Asthma - Penetrating chest injuries
2. Fistula formation
3. Gas-producing organisms (i.e. C. perfringes) |
|
|
Etiologies of acute pericarditis |
1. Serous - Chronic renal failure - Lupus/scleroderma - Tumor - Viral myocarditis
2. Fibrinous - Acute transmural MI - Chest radiation - Recent trauma or cardiac surgery - Dressler's syndrome - Progression form any of the serous processes
3. Supurative (end-stage of infectious process) |
|
|
Treatment of pericarditis |
1. Pain relief: NSAIDs, colchicine 2. Steroids, sparingly (can increase rate of recurrence) 3. Antibiotics/drainage for purulent pericarditis 4. Dialysis for uremic pericarditis |
|
|
Etiology of cardiac tamponade |
1. Acute - Trauma - Large LV rupture
2. Chronic: large effusion due to any etiology of pericarditis |
|
|
Clinical findings and their pathophysiologic explanation for cardiac tamponade |
1. Pulsus paradoxus: inspiration produces decreased systolic blood pressure.
2. Increased venous pressures --> systemic and pulmonary congestion
3. Decreased cardiac output (fatigue, etc.)
4. RA and RV collapse on imaging (LV is the last to be compressed, as it has the thickest wall). |
|
|
Cardiac tamponade: treatment |
Pericardiocentesis |
|
|
Constrictive pericarditis: definition and etiology |
Fibrosis and calcification resulting from healed pericarditis, often with adhesion formation between visceral and parietal pericardium and obliteration of the pericardial space.
Etiology: pretty much anything that can cause pericarditis |
|
|
Constrictive pericarditis vs. cardiac tamponade |
Same pathophysiology, with different characteristic findings:
UNIQUE FINDINGS TO CONSTRICTIVE PERICARDITIS: 1. Pericardial knock after S2 (abrupt cessation in filling due to rigidity of pericardial sac) 2. Kussmal's sign: JVD rise w/inspiration (same idea as pulsus paradoxus) 3. Pericardial thickening and calcification of cardiac silhouette on Xray 4. Pressure tracings: Atrial: Enhanced Y-descent Ventricle: Dip and plateau/square-root sign (upon cessation of filling)
FINDINGS UNIQUE TO TAMPONADE 1. Pulsus paradoxus 2. RA/RV compression |
|
|
Treatment of constrictive pericarditis |
Pericardial stripping: surgical lysis of adhesions |
|
|
Natural history of pericarditis |
1. Pleuritic chest pain; distinguished from angina with: - Sharp quality - Worse w/inspiration - Improvement on sitting - Characteristic radiation from precordium to trapezius
2. Fever
3. Compression of nearby structures: - Dysphagia (esophagus) - Hoarseness (RLN) - Hiccups (diaphragm)
4. Dyspnea due to pleural effusion/inflammation |
|
|
Physical exam findings of pericarditis |
1. Frictional rub; strongest in earlier stages of disease 2. Muffled heart sounds 3. Bronchial breathing and dullness to percussion due to lung compression |
|
|
Diagnosing pericarditis on echo |
Echo doesn't have great negative predictive value. "Pericarditis is a clinical diagnosis, not an echo diagnosis" |
|
|
Pressure tracings: acute vs. constrictive pericarditis |
Acute pericarditis: - Blunted Y-descent (less filling) - Equalized diastolic pressure in all four chambers and pericardium
Constrictive pericarditis: - Atria: steep Y-descent (stiff atrium empties into low-pressure ventricle) - Ventricles: square root sign |
|
|
Distinguishing pericarditis on EKG |
Important to distinguish from MI, because it involves diffuse ST elevation: - Unlike MI, there is no reciprocal ST depression from other leads (except aVR) - "Smiley face" betwen QRS and ST elevation (frowny face w/MI) |
|
|
Distinguishing cardiac tamponade on EKG |
1. Electrical alternans: alternating small and large QRS peaks 2. Low voltage 3. Sinus tachycardia |

