![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Atom Economy
|
The atom economy of a reaction is related to the efficiency of the reaction. The atom economy can be calculated by dividing the mass of atoms in the desired product by the mass of atoms in the reactants, and by multiplying by 100. For this reason, the atom economy displays the percentage of the reactants which are present in the desired products. Therefore, the higher the value, the more efficient (and less wasteful) the reaction is.
|
|
|
Cracking
|
When crude oil is distilled, the fractions that are produced can be cracked; this is where large hydrocarbon molecules are split into smaller, more useful hydrocarbon molecules. There are two types of cracking: catalytic and thermal. Catalytic cracking involves using zeolites as the catalyst, whilst thermal cracking involves applying high temperatures and pressures to split the hydrocarbon chains.
|
|
|
Fractional Distillation
|
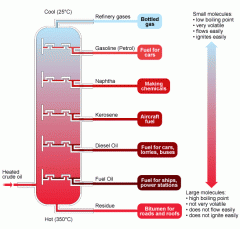
As substances within crude oil (such as petrol and diesel) all have different boiling points, they can be separated through fractional distillation. This then means that substance with the highest boiling point condense at the bottom, and the substance with the lowest boiling point condenses at the top.
Image source: http://www.bbc.co.uk/schools/gcsebitesize/science/images/5_fractional_distillation.gif |
|
|
Percentage Yield
|
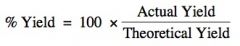
Theoretically, you should receive the same amount of a product as you have calculated. However, when experimented, this is usually not the case due to some of the product being lost, or the reactants may not fully react (or may react in a different way). For this reason, you can calculate the percentage yield by dividing the actual yield by the theoretical yield, and multiplying it by 100.
Image source: http://moodleshare.org/mod/page/view.php?id=8730 |
|
|
Reflux
|
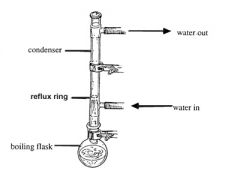
Reflux is the process in which the rate of a reaction is increased through the use of heat. Organic compounds; however, are very volatile with high vapour pressures. For this reason, a condenser is used to continually condense the vapours formed. This makes sure that the temperature of the reaction remains constant, and also ensures that the compounds do not become flammable and ignite.
Image source: http://www.chem.wisc.edu/areas/organic/orglab/tech/reflux.htm |
|
|
Addition Reaction
|
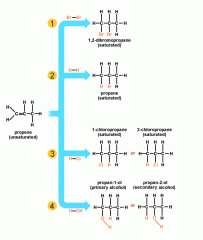
Addition reactions only occur with unsaturated compounds (alkenes or alkynes). Small molecules being added to the unsaturated compounds always add across the double bond, leaving you with a saturated compound. For example, if you were to add bromine to butene, you would end up with 1-2-dibromobutane.
Image source: http://www.bbc.co.uk/bitesize/higher/chemistry/carbon/reaction_carbon/revision/1/ |
|
|
Curly Arrow
|
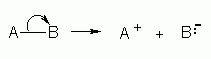
Curly arrows are used to show the movement of electron pairs and single electrons in organic reactions. When breaking a single bond, a curly arrow is used to show that the pair of electrons is being transferred the first atom and going to the second; this then leads to the first atom being positively charged, and the second element being negatively charged. When making bonds; however, a curly arrow is used to display that an electron pair of the property of the second atom is now being shared by both atoms.
Image source: http://homepages.abdn.ac.uk/che545/org/curl.shtml |
|
|
Dehydration Reaction
|
A dehydration reaction is a reaction in which water is removed from; an example of this is when ethanol vapours are passed over an aluminium oxide catalyst to form ethene and water.
|
|
|
Electrophile
|
Electrophiles are positively charged atoms which are attracted to negatively charged atoms. They are attracted to electrons, and accept an electron pair in order to bond to a nucleophile in chemical reactions.
|
|
|
Electrophilic Addition
|
An electrophilic addition reaction is a reaction in which the molecule is being attacked by an electrophile.
|
|
|
Elimination Reaction
|
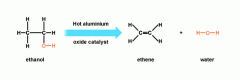
An elimination reaction is where the vapours of a saturated compound (alkene) is passed over a catalyst to form several products (including an alkene); an example of this is when the vapours of ethanol are passed over a hot aluminium oxide catalyst to form ethene and water.
Image source: http://www.bbc.co.uk/bitesize/higher/chemistry/carbon/reaction_carbon/revision/1/ |
|
|
Heterolytic Fission
|

Heterolytic fission is where a bond splits unevenly to form two ions of different charges. For example, if one atom were to gain two electrons, and the other atom didn't gain any, this would result in one of the atoms being positively charged, and the other being negatively charged.
Image source: http://hubpages.com/hub/AS-Chemistry-Things-You-Need-To-Know |
|
|
Hydrolysis
|
Hydrolysis involves breaking a bond in a molecule using water; an example of this is when water is added to sodium ethanoate. The water causes the chemical bonds to break, which then results in sodium and ethanoate ions. The ethanoate ions combine with the hydrogen atoms to form ethanoic acid.
|
|
|
Mechanism
|
A reaction mechanism is the sequence of reactions which lead to an overall chemical change. In organic chemistry, these mechanisms include addition, elimination, substitution, redox, and rearrangement reactions.
|
|
|
Nucleophile
|
A nucleophile is a chemical which is rich in electrons, and so is attracted to areas of positive charge. Examples of nucleophiles include chloride ions and ammonia.
|
|
|
Nucleophilic Substitution
|
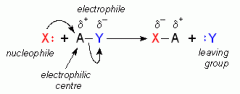
In nucleophilic substitution reactions, there is a formation of a new bond to the electrophile, and there is a breaking of the bond of the leaving group. The SN2 (bimolecular first step) mechanism is where the nucleophile bond forms and the leaving group breaks simultaneously. The SN1 (unimolecular first step) mechanism is where the leaving group breaks, and the nucleophile bond group forms.
Image source: https://people.ok.ubc.ca/wsmcneil/sn2sn1.htm |
|
|
Substitution Reaction
|
A substitution reaction is a reaction in which atoms in a molecule are replaced by other atoms; an example of this is when a bromine atom from bromoethane is displaced by a hydroxide ion.
Image source: http://www.chemguide.co.uk/mechanisms/nucsub/hydroxide.html |

