![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
119 Cards in this Set
- Front
- Back
|
G# P2112
|
G = # pregnancies
P: 2 = 2 live births 1 = 1 preemie 1 = 1 miscarriage/abortion 2 = 2 children living (so in this case, G=3) |
|
|
COMMON LABS:
Albumin |
3.2 - 5
g/dL made by liver negative acute phase reactant (levels will be lower in pt's suffering systemic inflammation, e.g. from infection or malignancy) LOW: hepatic impairment, poor nutrition, systemic inflammation, IV fluids HIGH: severe dehydration (high levels due to volume loss), pts on anabolic steroids may have actual hyperalbuminemia Symptoms: LOW - very low albumin may be accompanied by ascites & peripheral or pulmonary edema HIGH - asymptomatic |
|
|
COMMON LABS:
ALP |
Alkaline Phosphatase
33 - 131 IU/L HIGH: all forms of cholestasis (particularly obstructive jaundice) skeletal system disorders that involve hyperactive osteoblast activity, such as Paget's, fractures & malignant tumors MODERATE ELEVATION: Hodgkin's disease CHF intra-abdominal bacterial infections |
|
|
Alkaline Phosphatase & biliary tree obstruction
|
the response of the liver to any kind of biliary tree obstruction is to synthesize more Alkaline Phosphatase
|
|
|
COMMON LABS:
Ammonia |
10 - 80
mcg/dL HIGH - may be present in cases of hepatic encephalopathy, particularly when due to acute liver failure (e.g. viral hepatitis), but may not always be elevated in hepatic encephalopathy |
|
|
COMMON LABS:
bilirubin |
0.1 - 1.2
mg/dL Usually reported as total bilirubin, may be broken down into conjugated & unconjugated (conjugated is soluble & eliminated, unconjugated in insoluble and highly bound to albumin). Usually 90% unconjugated, 10% conjugated. High bilirubin with 90% or greater unconjugated is never from liver disease. May indicate excessive hemolysis or Gilbert's Syndrome (inherited inability to conjugate bilirubin) HIGH conjugated: liver disease |
|
|
COMMON LABS:
arterial pH venous pH |
ARTERIAL pH:
7.41 acidosis: 1 - 7.4 neutral: 7.41 alkalosis: 7.42 - 14 VENOUS pH: 7.36 acidosis may be from respiratory (lung disease, neuromuscular/diaphragm weakness) or metabolic (complicated, discussed elsewhere) sources alkalosis may be respiratory (hyperventilation) or metabolic (vomiting, burns, ingestion of base) |
|
|
COMMON LABS:
respiratory acidosis |
low pH (1 - 7.4 arterial)
high pCO2 (45+ arterial) normal or high-normal bicarb CAUSES: neuromuscular/diaphragm weakness lung disease (e.g. COPD) |
|
|
low pH
high pCO2 normal bicarb |
respiratory acidosis
CAUSES: neuromuscular/diaphragm weakness lung disease (e.g. COPD) |
|
|
COMMON LABS:
respiratory alkalosis |
high pH (7.42 or greater)
low pCO2 (<35) normal or high-normal bicarb CAUSES: hyperventilation from any cause |
|
|
high pH
low pCO2 normal bicarb |
respiratory alkalosis
CAUSES: hyperventilation from any cause |
|
|
metabolic alkalosis
|
high pH (7.42 or greater)
normal pCO2 high bicarb CAUSES: vomiting burns ingestion of base |
|
|
high pH
normal pCO2 high bicarb |
CAUSES:
vomiting burns ingestion of base |
|
|
COMMON LAB VALUES:
metabolic acidosis |
low pH (7.4 or less)
normal or low-normal pCO2 (35 - 45) low bicarb CAUSES when anion gap is normal (hyperchloraemia): loss of bicarb or ingestion of acid, e.g.: - diarrhea - renal tubular acidosis - Addison's disease - drugs (carbonic anhydrase inhibitors, e.g.) CAUSES when anion gap is HIGH: DKA lactic acid (e.g. shock or infection) drugs (metformin, salicylates, methanol) |
|
|
low pH
normal pCO2 high bicarb |
metabolic acidosis
|
|
|
anion gap
|
Useful in isolating cause of metabolic acidosis. In plasma, the sum of cations (Na + K) should be greater than anions (Cl + bicarb) by 6 - 18 mmol/L.
CAUSES when anion gap is HIGH (too much acid): M = methanol U = uremia D = DKA P = paraldehyde I = infection/ischemia/isoniazid L = lactic acidosis E = ethylene glycol, ethanol S = salicylates, starvation |
|
|
MUDPILES
|
pnemonic for causes of raised anion-gap metabolic acidosis
CAUSES when anion gap is HIGH (too much acid): M = methanol U = uremia D = DKA P = paraldehyde I = infection/ischemia/isoniazid L = lactic acidosis E = ethylene glycol, ethanol S = salicylates, starvation |
|
|
COMMON LABS:
pCO2 |
35 - 45 (arterial)
mmHg partial pressure of blood CO2 the balancing comoponent of the respiratory system (whereas the balancing component of the renal/metabolic system is bicarb/HCO3) higher pCO2: acidosis (remember, slow/shallow breathing retains CO2, so it makes sense that respiratory acidosis is caused by neuromuscular weakness or lung disease) lower pCO2: alkalosis (remember, faster/deeper breathing blows off CO2, and hyperventilation causes alkalosis) |
|
|
COMMON LABS:
pO2 |
80 - 100 arterial
mmHg NOT THE SAME as O2 sat LOW: hypoxemia |
|
|
COMMON LABS:
O2 Sat |
O2 Sat (arterial):
90 - 100% LOW: hypoxemia |
|
|
COMMON LABS:
HCO3 |
bicarb
19 - 26 mEq/L LOW: acidosis (metabolic) HIGH: alkalosis (metabolic) |
|
|
COMMON LABS:
BUN |
7 - 20
mg/dL Blood Urea Nitrogen: urea is the product of protein degradation; this is the most important catabolic pathway for elminating excess nitrogen HIGH: - Prerenal (cardiac decompensation, dehydration, high protein diet, muscle breakdown) - Renal (acute glomerulonephritis, chronic nephritis, tubular necrosis, kidney disease) - Postrenal (all types of obstruction of the urinary tract, such as stones, enlarged prostate, tumors) Usually interpreted based on a BUN:SCr ratio, normally 10 - 20. High ratio with normal SCr usually denotes prerenal causes. High ratio with high SCr is usually renal or postrenal. |
|
|
COMMON LABS:
Hemoglobin |
g/dL
MALE: 13.5 - 16.5 FEMALE: 12 - 15 |
|
|
COMMON LABS:
Hematocrit |
%
MALE: 41 - 50 FEMALE: 36 - 44 |
|
|
COMMON LABS:
RBC's |
million/uL
MALE: 4.5 - 5.5 FEMALE: 4 - 5 |
|
|
COMMON LABS:
Platelets |
100,000 - 450,000
|
|
|
COMMON LABS:
RDW |
(measure of the amount that RBC's vary in size)
10% - 15% HIGH: liver disease, anemia, B12 or folic acid deficiency LOW: macrocytic or microcytic anemia |
|
|
COMMON LABS:
MCV |
(Mean Corpuscular Volume: amount of space occupied by a RBC)
80 - 100 |
|
|
COMMON LABS:
CK |
(creatinine kinase: can detect rhabdomyolysis, serious muscle damage, or inflammation of the muscles)
CK-MB (found primarily in heart muscles): 0% - 3.9% CK-MM (found primarily in skeletal muscle): 96% - 100% CK-BB (found in brain, but when in blood it's primarily from smooth muscles including intestines, uterus, placenta): 0% |
|
|
COMMON LABS:
CPK |
(creatinine phosphokinase: another name for CK - total; again, indicates some sort of stress or injury to heart or other muscles)
8 - 150 IU/L |
|
|
COMMON LABS:
SCr |
0.5 - 1.4
mg/dL |
|
|
COMMON LABS:
calcium |
8.6 - 10.6
mg/dL |
|
|
COMMON LABS:
chloride |
95 - 110
mEq/L |
|
|
COMMON LABS:
magnesium |
1.5 - 2.5
mEq/L |
|
|
COMMON LABS:
phosphate |
2.5 - 4.5
mg/dL |
|
|
COMMON LABS:
potassium |
3.5 - 5
mEq/L |
|
|
COMMON LABS:
sodium |
135 - 145
mEq/L |
|
|
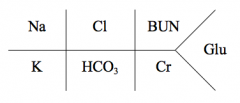
Na: 135 - 145
Cl: 95 - 110 BUN: 7 - 20 K: 3.5 - 5 HCO3: 19 - 26 Cr: 0.5 - 1.4 Glu (fasting): 60 - 110 Glu (2hrs postprandial): <140 |
|
|
The body responds to metabolic acidosis by ________.
|
trying to reduce the PCO2/[HCO3-] ratio, which is done by reducing the PCO2.
Reducing PCO2 is accomplished by increasing ventilation (which is characterized more by an increase in tidal volume than by an increase in respiratory rate). In its most pronounced clinical manifestation, this increase in ventilation is called Kussmaul Respiration. |
|
|
Kussmaul Respiration
|
The body responds to metabolic acidosis by trying to reduce the PCO2/[HCO3-] ratio, which is done by reducing the PCO2.
Reducing PCO2 is accomplished by increasing ventilation (which is characterized more by an increase in tidal volume than by an increase in respiratory rate). In its most pronounced clinical manifestation, this increase in ventilation is called Kussmaul Respiration. |
|
|
The body responds to respiratory acidosis by ________.
|
trying to increase serum [HCO3-], by 2 mechanisms:
1. rapid cell buffering (occurs within minutes) by intracellular buffers (Hemoglobin & proteins) 2. an increase in net acid excretion of NH4+ & carbonic acid (H2CO3), and bicarb resorption is increased (this response occurs over 3-5 days) |
|
|
In respiratory aacidosis, the elevation in PCO2 results from _____, never from ______.
|
reduced alveolar ventilation
increase in CO2 production (never due to this) |
|
|
The body's response to metabolic alkalosis is _______.
|
to increase PCO2, by lowering alveolar ventilation.
|
|
|
The body's response to respiratory alkalosis is __________.
|
to reduce the plasma HCO3, using 2 mechanisms:
1. rapid cell buffering (H+ ions from cells move into extracellular fluid, usually from intracellular hemoglobin, protein & phosphates) - response in minutes 2. decreasing renal acid excretion (ammonium & carbonic acid) |
|
|
Cubicin
|
daptomycin
|
|
|
Factive
|
gemifloxacin
|
|
|
Vibativ
|
telavancin
|
|
|
Teflaro
|
ceftaroline
|
|
|
Dificid
|
fidaxomycin
|
|
|
Amikin
|
amikacin
|
|
|
Garamycin
|
gentamycin
|
|
|
Mycifradin
|
neomycin
|
|
|
Myciguent
|
neomycin
|
|
|
Netromycin
|
netilmicin
|
|
|
Nebcin
|
tobramycin
|
|
|
Duricef
|
cefadroxil
|
|
|
Ancef
|
cefazolin
|
|
|
Ceclor
|
cefaclor
|
|
|
Mefoxin
|
cefoxitin
|
|
|
Cefzil
|
cefprozil
|
|
|
Ceftin
|
cefuroxime
|
|
|
Zinacef
|
cefuroxime
|
|
|
Omnicef
|
cefdinir
|
|
|
Suprax
|
cefixime
|
|
|
Claforan
|
cefotaxime
|
|
|
Vantin
|
cefpodoxime
|
|
|
Rocephin
|
ceftriaxone
|
|
|
Cefobid
|
cefoperazone
|
|
|
Fortaz
|
ceftazidime
|
|
|
Tazicef
|
ceftazidime
|
|
|
Fortum
|
ceftazidime
|
|
|
Tazidime
|
ceftazidime
|
|
|
Ceptaz
|
ceftazidime
|
|
|
Mazipime
|
cefepime
|
|
|
Noroxin
|
norfloxacin
|
|
|
Zymaxid
|
gatifloxacin
|
|
|
Zymar
|
gatifloxacin
|
|
|
Vigamox
|
moxifloxacin
|
|
|
Factive
|
gemifloxacin
|
|
|
Ketek
|
telithromycin
|
|
|
Nallpen
|
nafcillin (in dextrose_
|
|
|
Bactocill
|
oxacillin in dextrose
|
|
|
Bicillin LA
|
penicillin G benzathine
|
|
|
Bicillin CR
|
penicllin G benzathine + penicillin G procaine
|
|
|
Pfizerpen
|
penicillin G aqueous
|
|
|
Doryx
|
doxycycline
|
|
|
Vibramycin
|
doxycycline
|
|
|
Adoxa
|
doxycycline
|
|
|
Oracea
|
doxycycline
|
|
|
Alodox Convenience
|
doxycycline
|
|
|
Monodox
|
doxycycline
|
|
|
Ocudox
|
doxycycline
|
|
|
Morgidox
|
doxycycline
|
|
|
Oraxyl
|
doxycycline
|
|
|
Dynacin
|
minocycline
|
|
|
Solodyn
|
minocycline
|
|
|
Minocin
|
minocycline
|
|
|
Tygacil
|
tigecycline
|
|
|
Rosadan
|
metronidazole topical
|
|
|
Vandazole
|
metronidazole topical
|
|
|
Flagystatin
|
metronidazole + nystatin (vaginal)
|
|
|
Tindamax
|
tinidazole
|
|
|
Furadantin
|
nitrofurantoin (suspension)
QID |
|
|
Chloromycetin
|
chloramphenicol
|
|
|
Pentamycetin
|
chloramphenicol
|
|
|
Diochloram
|
chloramphenicol
|
|
|
Zyvox
|
linezolid
|
|
|
Rifadin
|
rifampin
|
|
|
Mycobutin
|
rifabutin
|
|
|
Xifaxan
|
rifaximin
|
|
|
Priftin
|
rifapentine
|
|
|
Lincocin
|
lincomycin
|
|
|
Synercid
|
quinupristin/dalfopristin
|
|
|
Which of the following medications would be an INAPPROPRIATE choice to sedate a patient with severe hypotension?
I. Haldol II. Versed III. Diprivan a. I only b. I and II c. II and III d. III only e. ALL |
D: Diprivan
Diprivan (propofol) would be a poor choice because it's primary effect on the cardiovascular system is to cause hypotension. Use with caution in patients who are hypovolemic, have abnormally low vascular tone (e.g. in sepsis), or are hemodynamically unstable. Hypotensive effect may be substantial. |
|
|
Diprivan
|
propofol
|
|
|
Versed
|
midazolam
|
|
|
Rocephin is usually dosed at _____, except in meningitis, when it is dosed _____.
|
Rocephin (ceftriaxone)
1g - 2g QD 2g BID in meningitis |
|
|
Rocephin
|
ceftriaxone
|
|
|
In which condition would Rocephin be dosed BID?
|
meningitis
|

