![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
91 Cards in this Set
- Front
- Back
|
Albumin plays an important role in the following ways:
|
1. Colloid osmotic pressure
2. Transport (of fatty acids, bilirubin) 3. Drug Transport (of aspirin, sulfonamides) |
|
|
What is the difference between plasma and serum?
|
Serum is the liquid part of blood AFTER coagulation, therefore devoid of clotting factors as fibrinogen. plasma is the liquid, cell-free (by centrifugation, for example) part of blood, that has been treated with anti-coagulants.
Think of serum = plasma - fibrinogen |
|
|
With the exception of albumin, all proteins contain _______________
|
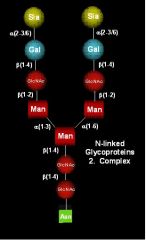
N-linked oligosaccharides ending with sialic acid bound to galactose
|
|
|
What happens during the lifetime of a plasma protein?
|
During the lifetime of the plasma protein, the terminal sialic acid residues are gradually chewed off by endothelial neuraminidases
After the loss of the sialic acid, the exposed galactose at the end of the oligosaccharide binds to an asialoglycoprotein receptor on the surface of hepatocytes, followed by receptor-mediated endocytosis and lysosomal degradation. |
|
|
If the serum electrophoresis shows a single intensity in the gamma globulin range, what does it mean?
|
The patient is suffering from monoclonal gammopathy. The single intensity means that only one immunoglobulin is in play while the remaining four are inactive.
|
|
|
Drugs in their free state are dangerous to the patient. Which protein is involved in inactivating drugs? How is it accomplished?
|
Albumin has multiple receptor sites on its surface through which it can bind to numerous drugs and thus inactivating them
|
|
|
In arteries, what does albumin provide?
|
Albumin, due to its large molecular weight, helps the arteries re-absorb and maintain water through its large colloid oncotic pressure
|
|
|
What is a drop in albumin concentration called?
What occurs during this period of time? |
Hypoalbuminemia
Water leaks out of the arteries and into the interstitial space (edema) |
|
|
What are the four abnormalities of albumin metabolism?
|
1. Reduced synthesis
Liver does not produce enough albumin because the patient is malnourished 2. Increased catabolism IL-6 3. Altered distribution Immune system components vasodilate arteries to fight infection which can lead to loss of albumin through the arterial wall 4. Abnormal losses Diseased kidneys will release protein (and albumin) into the urine |
|
|
What is the inherited form of hypoalbuminemia?
What is used to test it? How does it show itself in that test? |
Analbuminemia
* You can test it using protein-gel electrophoresis and find that this condition is marked by a missing albumin band near the cathode and an increase in the alpha 2 band. * The increase in the alpha 2 band shows compensation by the body for the missing Albumin |
|
|
It signifies that the patient is suffering from hypoalbuminemia because:
* The filled in circle is the missing albumin band * The arrow points to an increased band of alpha 2 proteins |
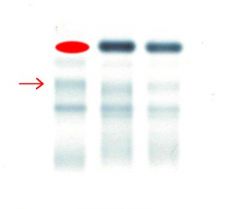
What does this protein-gel electrophoresis signify?
What is missing and what is elevated? |
|
|
What is it called when an individual has too much albumin protein?
|
Hyperalbuminemia
|
|
|
How does one become hyperalbuminemic?
|
1. Dehydration
2. Excessive stasis during venepuncture |
|
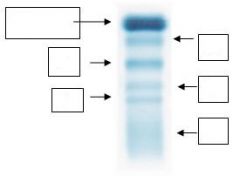
Name each of the bands of the serum electrophoresis
|
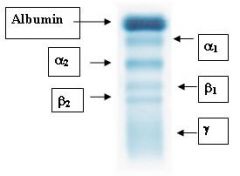
Look at the boxes for the answers
|
|
|
What proteins do you see in each of the electrophoresis bands?
α1-Globulins α2-Globulins β1-Globulins β2-Globulins γ-Globulins |
α1-Globulins: α1-antitrypsin, α-lipoprotein
α2-Globulins: haptoglobulin, ceruloplasmin, α2-macroglobulin β1-Globulins: transferrin, β-lipoprotein β2-Globulins: C3 (complement protein), fibrinogen (plasma), β2-microglobulin γ-Globulins: immunoglobulins (MADGE) |
|
|
Plasma proteins besides Albumin are specialized carriers of small molecules.
Name them and their function. |
1. PREALBUMIN and RETINOL-BINDING PROTEIN
* RETINOL-BINDING PROTEIN has retinol bound to it during its release from the liver * Retinol in turn binds to PREALBUMIN in the blood and is transported to other tissues 2. THYROXINE-BINDING GLOBULIN * The major transporter of thyroid hormones in the body * Binds to thyroxine with 100 times greater affinity than PREALBUMIN * However, PREALBUMIN'S congenital absence leads to abnormally low levels of thyroid hormones 3. CORTISOL-BINDING GLOBULIN (TRANSCORTIN) * Glucocorticoids are bound to this protein * Androgens and estrogens have their own sex hormone-binding globulin 4. HAPTOGLOBIN * Binds free hemoglobin released from erythrocytes with high affinity and thereby inhibits its oxidative activity. The haptoglobin-hemoglobin complex will then be removed by the reticuloendothelial system (mostly the spleen) 5. HEMOPEXIN * Binds to any free heme/hematin in the blood to prevent the loss of iron * The hemopexin-heme or hemopexin-hematin complex is removed in the liver |
|
|
If blood is seen in urine, what two conditions can we readily assume that the patient is suffering from?
How can we diagnostically detect this internal bleeding? |
We can assume that the patient is suffering either from HEMOGLOBINURIA or MYOGLOBINURIA
We can diagnostically detect HEMOGLOBINURIA by measuring the level of HAPTOGLOBIN -- we will see that this level is depressed. [Why will we see this?] The same cannot be said of MYOGLOBINURIA as HAPTOGLOBIN does not bind to the myoglobin released from muscles |
|
|
What is the function of α1-antitrypsin?
How does one go about measuring it in the blood? |
α1-antitrypsin is a serine protease inhibitor (serpin) and trypsin inhibitor (trypin). It protects tissue from enzymes released from inflammatory cells, especially elastase from WBC.
Its activity can be measured as the TRYPSIN INHIBITORY CAPACITY (TIC) |
|
|
What are the two types of alleles (normal and mutant) coding for α1-antitrypsin?
What conditions arise because of homozygosity of the mutant allele? |
Z is the mutant allele coding for α1-antitrypsin. As a result, homozygosity for the Z allele causes early-onset of pulmonary emphysema.
NOTE: α1-antitrypsin deficiency suggests that excessive proteolytic activity is an important mediator of tissue damage in chronic bronchitis and emphysema. Furthermore, these diseases then lead to hepatic disorders. |
|
|
Where is the activity of α1-antitrypsin seen?
|
Mostly in lung tissue
|
|
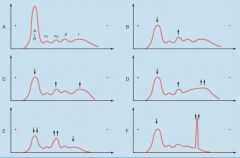
Please describe the diseases associated with each of the protein-gel electrophoreses.
|
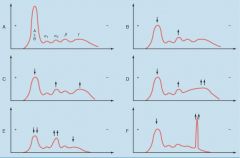
Plasma protein electrophoresis in various disease states. A, Normal. ALB, albumin. B, Immediate response pattern. C, Delayed response pattern. D, Liver cirrhosis. E, Protein-losing conditions (nephrotic syndrome, protein-losing enteropathy). F, Monoclonal gammopathy ("paraprotein").
|
|
|
If and when cell damage or cell proliferation occurs, we will see:
A. an increase in enzyme concentration in the blood B. a decrease in enzyme concentration in the blood |
A. an increase in enzyme concentration in the blood
|
|
|
Why are enzymes measured?
|
Most of the diagnostically useful serum enzymes are intracellular enzymes
that are released into the blood only when the cells get damaged. The enzymes are cleared from the circulation with half-lives of one to several days. A diagnostically useful enzyme should have higher or lower concentration due to a specific disease or condition. |
|
|
During what conditions do cells release enzymes?
|
1. Tissue necrosis: Myocardial infarction, hepatitis, acute pancreatitis.
2. Increased membrane permeability: Muscular dystrophy, dermato-myositis, angina pectoris. 3. Increased Tissue Source or Release from Tissue: Neoplasms, psoriasis, Paget’s disease, healing fractures. 4. Impaired Enzyme Excretion: duct obstruction, reduced urinary excretion |
|
|
How does one choose which enzyme test to administer?
|
* Has tissue damage occurred?
- If so, to what extent? * Which tissues have been damaged? |
|
|
What are isoenzymes?
|
They are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes usually display different kinetic parameters (i.e. different KM values), or different regulatory properties (pH or isoelectric point)
|
|
|
Name an example of a critical isoenzyme used in:
* Skeletal muscle * Cardiac muscle * Brain |
Creative Kinase
CK-MM (skeletal muscle) CK-MB (cardiac muscle) CK-BB (brain) |
|
|
What is the significance of α-fetoprotein?
|
Fetus: It is an immune system regulator but also a prenatal diagnostic for:
Down's syndrome -- low concentration Open neural tube -- high concentration Adults: It only appears in trace amounts in adults Good indicator/marker of HEPATOCELLULAR CARCINOMA |
|
|
Is copper an anti-oxidant or pro-oxidant?
|
Anti-oxidant
|
|
|
Ceruloplasmin can be found in low concentration and as such is a diagnostic tool for finding this disease.
|
Wilson's disease
|
|
|
What is a physical symptom of Wilson's disease?
|
The most characteristic symptom of Wilson's Disease is the Kayser-Fleisher ring – a rusty brown ring around the cornea of the eye that can best be viewed using an ophthalmologist’s slit lamp
|
|
|
What does C-reactive protein attack?
|
It attacks the pneumococcal cell wall of bacteria
|
|
|
What is scoline apnea?
|
The inability of CHOLINESTERASE to breakdown succinylcholine, an anesthetic, after surgery and thus lengthening the recovery period
|
|
|
Why would a surgeon want to measure a patient's recovery from succinylcholine administration?
|
If the patient has an allele which codes for little or no ChE activity, then the degradation of anesthetic is slow and thus dangerous to the patient
|
|
|
Which plasma cholinesterase allele codes for the silent protein (no activity)?
|
E1s
|
|
|
Graph A: Acute myocardial infartion (increased LDH1, LDH2)
Graph B: Acute hepatitis (increased LDH5) |

Looking at the lactate dehydrogenase levels for graph A and B above, what disease does each indicate?
|
|
|
What is the main component of Heme?
|
Protoporphyrin IX
|
|
|
What element is added to Protoporphyrin IX to yield Heme?
|
Iron (Fe2+)
|
|
|
What type of bonding is prominent with the Fe2+ in the heme group?
|
Coordinate covalent bonds (6 total/group)
|
|
|
Where in the protoporphyrin is the Fe2+ located?
|
In the center.
|
|
|
How many molecules of oxygen bind to each heme group?
|
1
|
|
|
When does the heme group no longer bind oxygen?
|
Interaction with oxygen oxidizes Fe (II) to Fe (III). Fe (III) in the heme group is called methemoglobin (which is useless in binding oxygen)
|
|
|
Where specifically does oxygen bind on the protoporphyrin?
|
Sixth ligand of Fe (II) out of the plane of the heme.
|
|
|
Name two primary functions of globin.
|
1. Modulate oxygen binding affinity
2. Make reversible oxygen binding possible |
|
|
Once globin surrounds the heme, what is the only component of the heme that is exposed to the solvent?
|
propionic acid side chains, which contribute to the solubility of hemoglobin
|
|
|
What is the fn of MYOglobin?
|
oxygen storage in muscles
It facilitates rapidly respiring muscle tissue |
|
|
What effect does detergent treatment have upon Mb?
|
heme and globin come apart, due to the breaking of hydrophobic interactions holding the two together.
|
|
|
What contributes to slow diffusion of oxygen from capillaries to tissue?
|
oxygen's solubility (Mb increases oxygen's solubility and facilitates oxygen diffusion)
|
|
|
Where is hemoglobin found?
|
RBC
|
|
|
What makes up the quarternary structure of hemoglobin?
|
2 alpha/beta dimers, a total of 4 polypeptide chains.
The association of the dimers contributes to the quarternary structure. |
|
|
Is the binding of oxygen to a free heme group reversible or irreversible?
|
Irreversible (enclosure in a protein allows reversible binding)
|
|
|
What basically is the reason that we see color differences between oxygenated and deoxygenated blood?
|
Oxygen binding alters heme electronic structure (changes in the electronic spectrum)
|
|
|
How is C reactive protein important diagnostically?
|
It is a good diagnostic for bacterial infection
|
|
|
Cyclooxygenase helps to create which proteins?
|
Prostaglandins
Thromboxane (TXA2) Prostacyclin (PGI2) |
|
|
What is the function of the following proteins?
* Prostaglandins * Thromboxane (TXA2) * Prostacyclin (PGI2) |
* Prostaglandin: Maintain normal hemostasis
* Thromboxane: a potent vasoconstrictor and inducer of platelet aggregation that functions by binding to receptors that function through the PLC-g pathway * Prostacyclin: a vasodilator and an inhibitor of platelet aggregation |
|
|
What inhibits cyclooxygenase activity?
By virtue of this, what other activities are also inhibited? |
Aspirin (COX-1 inhibitor) inhibits cyclooxygenase (COX) activity.
** Aspirin is an important inhibitor of platelet activation. By virtue of inhibiting the activity of cyclooxygenase, aspirin reduces the production of thromboxane (TXA2). Aspirin also reduces endothelial cell production of prostacyclin (PGI2), an inhibitor of platelet aggregation and a vasodilator. Localized to the site of coagulation is a balance between the levels of platelet derived TXA2 and endothelial cell derived PGI2. This allows for platelet aggregation and clot formation but preventing excessive accumulation of the clot, thus maintaining blood flow around the site of the clot. Endothelial cells regenerate active cyclooxygenase faster than platelets because mature platelets cannot synthesize the enzyme, requiring new platelets to enter the circulation (platelet half-life is approximately 4 days). Therefore, PGI2 synthesis is greater than that of TXA2. The net effect of aspirin is more in favor of endothelial cell-mediated inhibition of the coagulation cascade. This reflects the cardiovascular benefits to low dose administration of aspirin. |
|
|
Platelets will only adhere to this type of tissue?
|
Subendothelial tissue
|
|
|
Instead of directly attaching to collagen on the subendothelial tissue, platelets attach to this protein.
|
vWF
|
|
|
Of the following, which is produced by platelets and which by endothelial cells?
* Prostacyclin (PGI2) * Thromboxane (TXA2) |
Platelets: Thromboxane
Endothelial: Prostacyclin |
|
|
Activated platelets produce which proteins?
|
* ADP
* Thromboxane (TXA2) * Serotonin and Phospholipids |
|
|
vWF binds to this receptor on platelets.
|
Glycoprotein (GP) Ib-IX
|
|
|
What two pathways lead to the formation of a fibrin clot?
|
Intrinsic and extrinsic
|
|
|
What is the difference between the intrinsic and extrinsic pathways?
|
The formation of a red
thrombus or a clot in response to an abnormal vessel wall in the absence of tissue injury is the result of the intrinsic pathway. Fibrin clot formation in response to tissue injury is the result of the extrinsic pathway. |
|
|
Low levels and high levels of thrombin have these kinds of effects on the coagulation cascade.
|
Low thrombin levels activate V -> Va and VIII -> VIIIa
High thrombin levels deactivate Va and VIIIa |
|
|
How is thrombin created from the prothrombinase complex?
|
Within the prothrombinase complex, prothrombin is cleaved at 2 sites by factor Xa. This cleavage generates a 2-chain active thrombin molecule containing an A and a B chain which are held together by a single disulfide bond.
|
|
|
Thrombin plays a role in platelet aggregation and leukocyte adhesion AND impairment of plasminogen activation.
Specific the steps for each of these pathways. |
* Thrombin - PAR 1,2,3
IL1 and IL6 released Secretion of ICAM-1, VCAM-1 >> Platelet aggregation and leukocyte adhesion * Thrombin - TAFI Modulates degradation of fibrin clots Removal of C-terminal lysines Impairment of plasminogen activation and reducing rate of fibrinolysis |
|
|
In what ways is the activity of thrombin regulated?
|
1. Heparin - Antithrombin III [MOST IMPORTANT]
Complex leads to altered conformation and increased affinity for thrombin. It is specifically a SERINE PROTEASE INHIBITOR (serpin). Also, it inhibits factors IX, X, XI, and XII 2. alpha2-macroglobulin 3. alpha1-antitrypsin 4. Heparin cofactor II |
|
|
Fibrinogen consists of the following polypeptide chains.
|
Fibrinogen (factor I) consists of 3 pairs of polypeptides ([A-a][B-b][g])2
|
|
|
Is fibrinogen soluble or insoluble in the plasma? Why?
|
Soluble due to the fibrinopeptide A and B associated with the alpha and beta polypeptides
|
|
|
How does fibrin become insoluble in the plasma?
|
The fibrinopeptides, A and B, are cleaved prior to the formation of fibrin
|
|
|
Which factor involves the cross-linking of fibrin monomers?
|
Factor XIII
|
|
|
Factor XIII is able to cross-link fibrin monomers through this reaction.
|
Transglutaminase reaction: covalent linking between the glutamate and lysine residues on two different monomers
|
|
|
What are three activators of plasminogen to plasmin?
What are their specificities? |
1. tPA: In its inactive form, binds to fibrin and is activated at the clot.
[SPECIFIC TO CLOT] 2. Streptokinase: It is less selective than tPA, being able to activate circulating plasminogen as well as that bound to a fibrin clot. 3. Urokinase: Urokinase is produced as the precursor, prourokinase by epithelial cells lining excretory ducts. The role of urokinase is to activate the dissolution of fibrin clots that may be deposited in these ducts |
|
|
What two inhibitors are there for plasminogen to plasmin?
|
1. α2-antiplasmin
2. PAI 1,2 (plasminogen activator inhibitors) |
|
|
Which bleeding disorder is associated with prolonged bleeding?
Which bleeding disorder is associated with prolonged coagulation? |
Factor VIII: Prolonged bleeding
Factor IX: Prolonged coagulation |
|
|
Affected individuals suffer easy bruising, prolonged bleeding from wounds and
muscle hemorrhage, what disorder am I? |
Hemophilia A
|
|
|
I am caused by deficiency of vitamin K-dependent procoagulant protein Factor IX, what disorder am I?
|
Hemophilia B
|
|
|
It also has a role in stabilizing and increasing plasma concentration of Factor VIII, what factor am I?
|
vWF
|
|
|
Presentation is in bleeding from the umbilical cord
stump in neonates, intracranial hemorrhage, or after surgical challenge in children (often dental extractions), what disorder am I? |
Fibrinogen and Factor XIII
|
|
|
The platelet plug is normally formed with this factor in place.
|
vWF
|
|
|
What is contact activation?
|
When blood makes contact with negatively charged surfaces it triggers a series of interactions that involve factor XI, prekallikrein and high molecular weight kininogen leading to blood coagulation
|
|
|
Deficiency in factor __?__ confers an injury-related bleeding tendency.
You also witness reduced contact activation. |
XI
|
|
|
These factors require liver Vitamin K for their biosynthesis.
|
Factors II, VII, IX, and X
|
|
|
The membrane and calcium associations of these enzymes depend on post-translational
modification of γ-carboxylation of glutamic acids. |
Precursors of factors II, VII, IX, and X
|
|
|
This drug interferes with blood clotting by inhibiting Vitamin K-dependent biosynthesis of the clotting factors
|
Warfarin
|
|
|
Warfarin is recognized as a _______?_______
|
Anti-coagulant
|
|
|
This lab test tests the function of the 'extrinsic' and final common clotting pathways.
|
Prothrombin Test (PT)
|
|
|
This lab test tests the function of the 'intrinsic' and final common clotting pathways
|
Activated partial thromboplastin time (APTT!)
|
|
|
What factors does PT measure?
|
Factor VII and common pathway factors (I, II, V, and X)
|
|
|
What factors does APTT measure?
|
Factors VIII, IX, XI, and XII and common pathway factors (I, II, V, and X)
|
|
|
Diagnosing myocardial infarction has previously followed the serum levels of CK, AST, and LDH.
Recently however, this enzyme has also been measured alongside CK. |
Troponin I
|

