![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
Ore |
Mineral from which it is possible to produce metal using technology that already exists or can be reasonably developed |
|
|
Mineral |
Homogeneous solid boy, formed by natural processes, that has regular atomic arrangement which sets limits to its range of chemical composition and gives it characteristic physical properties
Form through geological processes or alongside biologic processes |
|
|
Class I |
Native elements -Pure elements -Gold (Au), Copper (Cu), Silver (Ag), Diamond (C), Sulphur (S) |
|
|
Class II |
Sulphides -Sulphur is the anion -Pyrite (FeS2), Chalcopyrite (CuFeS2), Chalcocite (Cu2S), Bornite (Cu5FeS4), Sphalerite (ZnS), Molybdenite (MoS2)
|
|
|
Class III |
Halides -Anions are halogens; cations are usually alkali (group 1) or alkaline (group 2) earth elements -Halite (NaCl), Fluorite (CaF2), Silvite (KCl)
|
|
|
Class IV |
Oxides & Hydroxides -Oxides contain oxygen as anion; Hydroxides contain OH- as cation -(Oxides) Magnetite (Fe3O4), Hematite (Fe2O3) -Hydroxides: Brucite (Mg(OH)2), Goethite (FeOOH) |
|
|
Class V |
Carbonates, Nitrates, Borates -Tetrahedral structure -(Carbonates) Calcite (CaCo3), Magnesite (MgCo3), Siderite (FeCO3) -(Nitrate) Nitratine (NaNO3) -(Borate) Borax (Na3B4O7 . 10H2O) |
|
|
Class VI |
Sulphates, Chromates, Molybates, Tungstates SO4[2-] CrO4[2-] MoO4[2-] WO4[2-] (^Tetrahedral structure) -(Sulphates) Anhydrite (CaSO4), Gypsum (CaSO4 . 2H2O) -(Chromate) Crocite (PbCrO4) -(Molybdate) Wulfenite (PbMoO4) -(Tungstate) Scheelite (CaWO4) |
|
|
Class VII |
Phosphates, Arsenates, Vanadates -Trivalent tetrahedral polyanions (PO4[3-], AsO4[3-], VO4[3-]) -(Phosphates) Apatite (Ca5(PO4)3(OH)) -(Arsenates) Scorodite (Fe[3+]AsO . 2H2O) -(Vanadates) Vanadinite (Pb5(VO4)3Cl) |
|
|
Class VIII |
Silicates (largest class) -All silicates contain SiO4[4-] -Quartz (SiO2), Potassium feldsar group (KAlSi3O8), Plagioclase feldspar group (NaAlSi3O8), Sodalite (Na4Al3(SiO4)3Cl), serpentine group
*Oxygen (46.6% ) and silicon (27.7%) are most common elements in Earth's crust
|
|
|
Silicates: sub-classes |
-Nesosilicates (single tetrahedrons) -Sorosilicates (double tetrahedrons) -Inosilicates (single & double chains) -Cyclosilicates (rings) -Phyllosilicates (sheets) -Tectosilicates (frameworks)
Acronym: Never Say I Can't Pull Tonight |
|
|
Class IX |
Organic Minerals -Created in a geological setting and have organic chemicals in their composition -These chemicals can be the result of biological activities -Amber (C10H16O), whewellite (kidney stones )
|
|
|
Ore Minerals |
Use: metals extraction
Examples: chalcopyrite, galena, sphalerite |
|
|
Industrial Minerals |
Use: specific industrial applications
Examples: kaolin for paper; garnet for abrasives |
|
|
Energy Minerals |
Use: power generation
Examples: coal *This is a rock, not a mineral* |
|
|
Gemstones |
Use: jewellery
Examples: diamond (also has industrial applications), tourmaline, emerald
|
|
|
Gangue Minerals |
Use: waste, or used for bricks
Examples: clay minerals, quartz, feldspars |
|
|
Colour |
Subjective; should only be used to complement other mineral properties |
|
|
Streak |
Colour of powdered form of mineral |
|
|
Lustre & transparency |
Lustre: how the mineral reflects light -adamantine, dull, metallic, sub-metallic, greasy, pearly, silky, resinous, vitreous, waxy
Transparency: opaque, translucent, or transparent -> birefringence |
|
|
Crystal Habit |
External shape of a crystal; reflects internal structure. 3 general terms: Euhedral = very well developed Subhedral = not very well developed Anhedral = very poorly developed
(Also: columnar, blocky, granular, tabular, fibrous, prismatic, globular, foliated, dendritic) |
|
|
Cleavage & fracture |
Cleavage = tendency of a mineral to break in certain directions when subject to stress on a particular plane: perfect, good, poor, none + number of cleavage planes
Fracture: chipping of a mineral. Rough or conchoidal (eggshell) |
|
|
Hardness |
Mohs Hardness Scale (Talc is softest at 1; diamond is hardest at 10) Fingernail = 2.5 Glass = 5.5 Steel = 7 Example: mineral isn't scratched by fingernail but it also doesn't scratch glass: 2.5 < x < 5.5 |
|
|
Tenacity |
Mineral's reaction to stress -Brittle -Sectile -Malleable -Ductile -Flexible
|
|
|
Magnetism |
Ferromagnetic = high magnetism Paramagnetic = little magnetism Diamagnetic = slightly repelled by a magnet
Magnetic minerals typically lose magnetism when even slightly altered |
|
|
Specific Gravity |
Similar to density (can be estimated using heft test). Density = ratio of mass to volume
G = (M - P) / (W + (M - P) - S)
G = specific gravity; P = weight of picnometer; M = weight of sample + picnometer; W = weight of picnometer full of water; S = weight of sample + picnometer + water
|
|
|
Other Mineral Identification Tests |
Reaction to acid, behaviour under fluorescent light, radioactivity, smell, tastes |
|
|
Grade |
Concentration (%) of metal or mineral of interest in a sample
Grades of precious metals are usually given in ppm = g/tonne (ppb = mg/tonne = 0.0001% for very low grades) |
|
|
Distribution |
Weight of the metal or mineral of interest in a product divided by the weight of the metal or mineral of interest in the feed
% of metal or mineral of interest in the product (same as recovery)
|
|
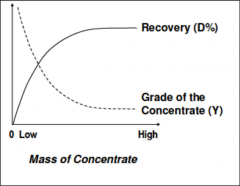
|
Ideal: less mass of concentrate and high recovery but this is not common
Engineering decision: where is our target in the curve? Depends on many factors
How little mass do we want in concentrate in detriment of recovery? |
|
|
Beneficiation |
One of a variety of processes that take extract ore from mining and separate it into the desirable mineral and gangue |
|
|
Mineral Factors |
Physical and chemical properties of a mineral that determine the beneficiation process to be selected and the performance in industrial processes
Must be assessed for a specific mineral in a specific deposit: all deposits are unique |
|
|
Mineral Factors in processing: Examples |
Texture: intergrowths and aggregates can make valuable mineral inaccessible to reagents Colour: Sorting minerals Crystal structure: affects reactivity to leaching solutions. Habit can affect possible uses Particle size: affects screening or settling in liquid Cleavage & fracture: how minerals break influences separation procedures Mineral associations: it is often impossible to liberate ore mineral from intergrowths and inlcusions with gangue Hardness & tenacity: play strong roles in mineral processing, especially grinding. Harder and more malleable minerals require more energy to grind Magnetism: minerals with magnetic properties can be separated from diamagnetic gangue after liberation Specific gravity: flotation and settling velocity: two minerals with the same settling velocity in a liquid can't be separated by flotation in that liquid Mineral surface: oxidation of surface affects floatability Porosity: influences reagent consumption in flotation Impurities or inclusions: amount of impurities or inclusions in a mineral deposit determines quality of products Alteration products: affect beneficiation processes. (HFOs + clay minerals form thin layer on gold particles that prevents flotation) |

