![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
1317 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
a Substance
|
A material with uniform properties throughout
e.g. salt, steel, gold, water. |
|
|
|
Mass
|
A measure of the amount of matter in an object.
|
|
|
|
a Physical Property
|
of a substance is its density.
|
|
|
|
Density
|
the mass of an object made of the substance divided by it's volume.
|
|
|
|
Density formula
|
D=m/V
|
|
|
|
Physical Properties of substance: (6)
|
- color
- hardness - ductility - resistivity - viscosity - solubility |
Capable
Handy Derelicts Read Verizon Successfully |
|
|
Chemical Properties describe...
|
the ability of a substance to be changed into new substances
|
|
|
|
Phases of matter
|
Sold, liquid, and gas
|
|
|
|
describe a Solid
|
has a definite shape and volume
|
|
|
|
describe a Liquid
|
has definite volume and assumes the shape of its container
|
|
|
|
describe a Gas
|
will spread out to occupy the entire space of whatever container it's in.
|
|
|
|
Kinetic Theory of Matter states:
|
all matter consists of atoms or molecules in a state of constant motion.
|
|
|
|
a Plasma is:
|
a partially ionized gas in which some of the electrons are not bound to any atoms or molecules.
|
|
|
|
Plasmas are... (2 points)
|
electrically conductive and can be generated inside vacuum tubes with a beam of electrons
are the most common form of matter in the universe b/c stars are composed of plasmas. |
|
|
|
Physical Properties of a Gas: (4)
|
Temperature (T)
Volume (V) Mass (n), and Pressure (P) |
|
|
|
Lowest Possible Temperature (Celsius and Kelvin)
|
-273.15 Celsius
0 K |
|
|
|
What determines temperature of a gas?
|
the average kinetic energy of the atoms or molecules of the gas
|
|
|
|
Mass of gas is measured in?
|
Moles (n)
|
|
|
|
a Mole of gas can be defined as...?
|
the number of molecules in the gas expressed in terms of Avogadros number
|
|
|
|
Avogadro's number?
|
6.02 x 10²³
|
|
|
|
the Pressure of a gas...
|
is the force the gas exerts on a wall of the container divided by the area of the wall
|
|
|
|
Pressure of a gas on a wall (formula)
|
P=F/A
|
|
|
|
SI unit of force is...
|
the newton (N)
|
|
|
|
SI unit of pressure is... (4 of them)
|
the pascal (Pa)= 101,325pa = 1 atm
the atmosphere (atm): 1 atm the millimeters of mercury (mm Hg): 760mm the pounds per square inch (lb/in₂, psi)=14.7 |
|
|
|
The Ideal Gas Law is based on...
|
the assumption that there are no forces acting between the molecules of the gas.
|
|
|
|
The ideal gas law (formula)
|
PV=nRT
P (pressure) V (volume) n (mass) R (universal gas constant) T (temperature) |
|
|
|
Three (3) laws that rewrite the Ideal Gas Law
|
Boyle's
Charle's Gay-Lussac's |
|
|
|
Boyle's law, as it relates to the ideal gas law
|
the temperature is constant and pressure and volume are inversely related.
|
|
|
|
Charle's law, as it relates to the ideal gas law
|
pressure is constant and volume and temperature are directly related
|
|
|
|
Gay-Lussac's law, as it relates to the ideal gas law
|
Volume is constant
|
|
|
|
In terms of gas law, adding or removing heat...
|
will change the pressure, volume, and temperature of a gas.
|
|
|
|
The atomic theory of matter was first suggested by:
|
a Greek named Democritus.
|
|
|
|
Who expanded Democritus' idea of matter and when?
|
John Dalton, 1780s.
|
|
|
|
John Dalton's atomic model: (4 points)
|
- Matter is made up of atoms.
- Atoms of an element are similar to each other. - Atoms of different elements are different from each other. - Atoms combine with each other to form new kinds of compounds. |
|
|
|
William Crokes created what? when? how?:
|
1870s; cathrode rays in a vacuum by connecting a high voltage battery to anode and cathode
|
|
|
|
J.J. Thompson discovered what? when? relevancy?
|
1896; showed that cathode rays were composed of negatively charged particles with a mass almost 2 thousand times smaller than the mass of the hydrogen atom.
Thus: Thompson discovered the electron. |
|
|
|
What model of the atom did Thompson develop?
|
The Plum Pudding model
|
|
|
|
The Plum Pudding Model (2 points)
|
the atom consists of electrons equally mixed in a sphere of positive material.
The electrons were the plums and the positively charged matter was the pudding. |
|
|
|
Radioactivity was discovered when?
|
1896;
|
|
|
|
Ernest Rutherford tested what?
|
Thompson's model
|
|
|
|
Rutherford's model of the atom (4 points)
|
1. Most of an atom consists of empty space.
2. At the center of the atom is a nucleus that contains most of the mass and all of the positive charge of the atom. 3. The region of the space outside the nucleus is occupied by electrons. 4. The atom is neutral because the positive charge on the nucleus equals the sum of the negative charges of the electrons. |
|
|
|
Niels Bohr model of the atom was developed when? And, was based on: (2 points)
|
1913;
based on recent discoveries in quantum mechanics and the discrete spectrum of light emitted by hydrogen atoms. |
|
|
|
Niels Bohr model of the atom: (3 points)
|
1. Electrons orbit the nucleus, but only in discrete orbits or energy levels.
2. Electrons don't emit radiation when orbiting the nucleus. 3. When an electron moves from an outer orbit or higher energy level to an inner orbit or lower energy level, it emits a photon with an energy equal to the energy difference. |
|
|
|
What is a compound? (2 points)
|
two or more elements that have been chemically combined.
There is always a ratio of elements. |
|
|
|
What is an element?
|
a substance that can't be broken down into other substances.
|
|
|
|
What is a molecule?
|
the smallest particle of substance that can exist independently and has all of the properties of that substance.
|
|
|
|
What is a mixture?
|
any combination of two or more substances in which the substances keep their own properties.
|
|
|
|
Some common compounds: (4)
|
Acid
Bases Salts Oxides |
|
|
|
What is an acid?
|
An acid contains hydrogen ions (H⁺)
Have a sour taste. |
|
|
|
What is a base?
|
All bases contain hydroxl ions (OH⁻)
Bases have a bitter taste. Are in many cleaning products. |
|
|
|
What's an indicator?
|
a substance that changes color when it comes in contact with an acid or a base.
e.g. litmus paper: blue turns red in an acid; red turns blue in a base. |
|
|
|
What does neutral mean?
|
a substance that is neither acid nor base.
|
|
|
|
How are salts formed? What is a byproduct?
|
Salt is formed when an acid and a base combine chemically. Water is also formed.
|
|
|
|
What is neutralization?
|
When an acid and a base combine chemically and a water is formed.
|
|
|
|
What is an oxide?
|
a compound formed when oxygen combines with another element.
|
|
|
|
What is a chemical reaction?
|
When two or more elements or compounds react to one another, and one or more substances are formed.
|
|
|
|
What happens in exothermic chemical reactions?
|
Energy is released.
|
|
|
|
What happens in endothermic chemical reactions?
|
Energy is required.
|
|
|
|
What happens in a chemical equilibrium?
|
it occurs when the quantities of reactants and products are no longer changing, but the reaction may still proceed forward and backward.
|
|
|
|
What can be said of the rate of reaction in a chemical equilibrium?
|
The rate of forward reaction must equal the rate of backward reaction and the reaction is said to be in a steady state.
|
forward
backward steady |
|
|
A chemical equation represents what?
|
the reactants and products of a reaction.
The mass of the reactants is equal to the mass of the products. i.e. same number of atoms on both sides. |
|
|
|
Protons are...
|
- positively charged
- 2000 times the mass of of an electron - the magnitude of its charge is the same as an electron. |
|
|
|
Atomic number is...
|
The number of protons in the nucleus
|
|
|
|
an atom's charge is what? why?
|
neutral, because the number of electrons equals the number of protons.
|
|
|
|
Neutrons, compared to protons, are...
|
are slightly more massive, and have no charge.
|
|
|
|
Isotopes of an element...
|
have the same number of protons (atomic number) in the nucleus, but differ in atomic mass (number of nucleons).
|
|
|
|
Atomic mass units
|
The mass of an element on the periodic table
|
|
|
|
Mass number of an atom is the...
|
sum of its protons and neutrons
|
|
|
|
At what levels do electrons, orbiting the nucleus, occupy?
|
Quantized or discrete levels.
|
|
|
|
The electrons closest to the nucleus have what amount of energy?
|
the least amount
|
|
|
|
According to the Pauli exclusion principle...
|
Electrons in an atom all have to be different, i.e. have different quantum numbers.
|
|
|
|
At higher energy levels, electrons can have more...
|
angular momentum
|
|
|
|
In electrons, having more angular momentum is having more...
|
quantum numbers
|
|
|
|
What energy levels are filled first in atoms?
|
The lowest levels or energy.
|
|
|
|
How are elements in the periodic table built?
|
by adding protons, neutrons, and electrons to the hydrogen nucleus.
|
|
|
|
Atoms react with each other when...
|
their outer levels are unfilled.
|
|
|
|
Which elements always have their outer energy levels filled?
|
the inert gases
|
|
|
|
Electrons move to higher energy levels when...
|
they gain energy by absorbing a photon or by a collision.
|
|
|
|
An electron cannot leave one level until...
|
it has enough energy to reach the next level.
|
|
|
|
What are excited electrons?
|
are electrons that have absorbed energy and have moved farther way from the nucleus.
|
|
|
|
The Periodic Table of Elements is...
|
an arrangement of the elements in rows and columns, so that it's easy to locate elements with similar properties.
|
|
|
|
the periods of the PTE, are...
|
the horizontal rows of the table.
|
|
|
|
the vertical columns of the PTE, are called...
|
groups or families
|
|
|
|
Elements in family have...
|
similar properties
|
|
|
|
There are three types of elements...
|
1. metals
2. non-metals 3. metalloids |
|
|
|
With the exception of hydrogen, all elements in Groups 1 can be called...
|
alkali metals
|
|
|
|
Common physical properties of Group 1 elements are...
|
- shiny
- softer & less dense than other metals - the most chemically active |
|
|
|
Group 2 elements can be called...
|
Alkaline earth metals
|
|
|
|
Alkali Earth metals (group 2 metals) can be described as being...
|
- harder
- denser - have higher melting points - are chemically active |
|
|
|
Elements found between Period 4 to 7, under Group 4-12 can be categorized as...
|
transition elements
|
|
|
|
Transitions elements (period 4-7, groups 4-12) can be described as...
|
- hard
- have high melting points - compounds are colorful |
|
|
|
Nonmetals are not easy to recognize as metals because...
|
they do not always share physical properties
|
|
|
|
The general properties of nonmetals are...
|
- dull
- brittle - not good conductors or heat/electricity |
|
|
|
Nonmetals include these states of matter:
|
- Solid
- Liquid (1, bromine) - Gas |
|
|
|
What's the range of number of electrons that nonmetals can have in their outermost energy levels? And the result of this?
|
4-8 electrons
The outer levels are usually filled with eight electrons. |
|
|
|
The outstanding chemical property of nonmetals is they...
|
react with metals
|
|
|
|
In terms of chemistry, the difference in the number of _______ is the cause of the differences between _____ and ____?
|
electrons;
metals, nonmetals |
|
|
|
Halogens can be found in group ____?
|
17
|
|
|
|
Halogens combine readily with _____ to form _____?
|
metals, to form salts
|
|
|
|
Describe metalloids
|
They have properties in between metals and nonmetals.
|
|
|
|
Physical properties of metalloids
|
1. Solids having the appearance of metals.
2. White or gray, but not shiny 3. conduct electricity, but not as well as a metal. |
|
|
|
Chemical properties of metalloids
|
1. all have some characteristics of metal and nonmetals.
2. their properties do not follow patterns like metals and nonmetals. Each must be studied individually. |
|
|
|
Metalloids are found between what two groups?
|
Group 13 to 16.
They do not occupy the entire group. |
|
|
|
Which metalloid is a semi-conductor?
|
Silicon
|
|
|
|
Describe semiconductors
|
has a conductivity between that of an insulator and a conductor.
|
|
|
|
What are valence electrons?
|
- The outermost electrons in atoms.
- They are the only electrons involved in the bonding process. - They determine properties of the element. |
|
|
|
A chemical bond is...
|
a force of attraction that holds atoms together.
|
|
|
|
When atoms are chemically bonded, they cease to...
|
they cease to have their individual properties.
|
|
|
|
A covalent bond is formed when...
|
2 atoms share electrons in order to get completely filled shells.
|
|
|
|
Covalent bonding happens between...
|
nonmetals
|
|
|
|
Covalent compounds are...
|
compounds whose atoms are joined by covalent bonds.
|
|
|
|
An ionic bond is...
|
a bond formed by the transfer of electrons from one atom to the other.
|
|
|
|
Ionic bonds happen when...
|
Metals and nonmetals bond.
|
|
|
|
Ions are...
|
atoms with an unequal number of protons and electrons.
|
|
|
|
To determine whether an ion is positive or negative...
|
compare the number of protons (+charge) to the electrons (- charge).
|
|
|
|
Ionic compounds are...
|
Compounds that result from the transfer of metal atoms to nonmetal atoms.
|
|
|
|
Metalic bonding exists..
|
only in metals.
|
|
|
|
In metals, the electrons are not...
|
fixed to any particular nuclei and are free to move.
|
|
|
|
Hydrongen bonding is an example of...
|
a force that acts between two molecules of a liquid or a solid and holds the molecules together.
|
|
|
|
An example of hydrogen bonding is...
|
the water molecule
|
|
|
|
What does it mean when a bond is identified as polar?
|
When the total charge of a molecule is zero, and, when one end of the molecule (an atom) is negatively charged and the other end (the other atom) is positively charged.
|
|
|
|
Hydrogen bonds can occur...
|
within and between other molecules.
For example: 2 strands of DNA; water molecules and amino acids of proteins are involved in maintaining the protein's proper shape. |
|
|
|
In a composition reaction...
|
two or more substances combine to form a compound.
|
|
|
|
Formula of a composition reaction:
|
A + B ---> AB
|
|
|
|
In a decomposition reaction...
example? |
a compound breaks down into two or more simpler substances.
Ex: electric current splits water molecules into hydrogen and oxygen gases. |
|
|
|
Basic formula of a decomposition reaction:
|
AB ----> A + B
|
|
|
|
In a single replacement reaction...
example? |
a free element replaces an element that is part of a compound.
Ex: Iron plus copper sulfate yields iron sulfate plus copper. |
|
|
|
Basic formula of a single replacement reaction:
|
A + BX ---> AX + B
|
|
|
|
In a double replacement reaction...
example? |
parts of two compounds replace each other.
Ex: Sodium chloride plus mercury nitrate yields sodium nitrate plus mercury chloride. |
|
|
|
Basic formula for a double replacement reaction?
|
AX + BY ---> AY + BX
|
|
|
|
Dynamics is the study of...
|
the relationship between motion and the forces affecting motion.
|
|
|
|
Forces cause...
|
objects to move and can be understood as a push or a pull.
|
|
|
|
Gravity is...
|
the force that causes objects to fall to Earth.
|
|
|
|
The universal law of gravity states...
|
that there is a gravitational attraction between all objects on Earth.
|
|
|
|
Universal Law of Gravity equation:
|
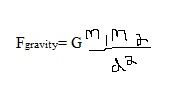
Where:
G = the universal gravitational constant d= is the distance between the two masses (m) |
|
|
|
Coulomb's Law
|
Electrostatic forces between objects are attractive when the charges are different and repulsive when they are the same.
It's what keeps electrons rotating around the nucleus in atoms. |
|
|
|
Coulomb's Law explains what kind of bonding?
|
ionic
|
|
|
|
Unlike charges, magnetic forces are...
|
polar; poles come in pairs.
|
|
|
|
When a charge is stationary, it produces an _______ (2 points)
|
an electric field around it.
|
|
|
|
When a charge is moving, it produces ________ (2 points).
|
circular magnetic fields that are perpendicular to the direction of motion.
|
|
|
|
The existence of nuclear forces is apparent from the fact that...
|
the repulsive electrostatic force between protons does not drive the protons apart.
|
|
|
|
When protons are very close to each other, there is a(n)....
|
attractive force called the strong force.
|
|
|
|
Neutrons act as a ______ in a nucleus? Doing what for the atom?
|
glue; making it more stable
|
|
|
|
The lack of stability in large nuclei can be understood from the fact that all....
|
protons in a nucleus are repelling each other, and nuclear forces only affect the adjacent proton or neutron.
|
|
|
|
Statics is the study of...
|
physical systems at rest or moving with a constant speed.
|
|
|
|
Static force occurs when...
|
the net force acting on an object is zero.
|
|
|
|
In terms of static force, describe what's going on when a book is resting on a table.
|
When a book is resting on a table, the force of gravity is in equilibrium with the force of the table acting upward on the book. The force of the table on the book is called normal force.
|
|
|
|
Static friction describes...
|
the force of friction of two surfaces that are in contact but don't have any motion relative to each other, such as a block sitting on an inclined plane.
|
|
|
|
Kinetic friction describes...
|
the force of friction of two surfaces in contact with each other when there is relative motion between the surfaces.
|
|
|
|
4 rules of Static & Kinetic friction
|
1. The materials that make up the surfaces determines the magnitude of the frictional force.
2. The frictional force is independent of the area of contact between the two surfaces. 3. The direction of the frictional force is opposite to the direction of motion for kinetic friction. 4. The frictional force is proportional to the normal force between the two surfaces in contact. |
|
|
|
Newton's first law of motion
|
an object at rest will remain at rest and an object in motion will remain in motion at a constant velocity unless acted upon by an external force.
This is also the law of inertia and can be derived from Newton's second law. |
|
|
|
Newton's second law of motion
|
if a net force acts on an object, it will cause the object to accelerate.
|
|
|
|
Newton's Third Law of motion
|
forces on objects come from other objects and hence always exists in pairs.
The force on object 1 on object 2 is equal and opposite to the force of object 2 on object 1. |
|
|
|
Newton's 3rd law of motion lead mathematically to... and which is...
|
the law of conservation of momentum, which governs a collision or any interaction between two objects.
|
|
|
|
Momentum is (describe equation)
|
The mass of an object times its velocity.
|
|
|
|
When there is a collision between two objects, the momentum...
|
before the collision is equal to the momentum after the collision.
|
|
|
|
Which of Newton's law leads to the concept of work?
|
Newton's 2nd law.
|
|
|
|
Work is...
|
force acting through a distance
|
|
|
|
Energy is...
|
the ability to do work
|
|
|
|
Kinetic energy is...
|
the energy of motion.
|
|
|
|
Potential energy is...
|
when the force depends on the relative locations of the objects, as is the case of electric and gravitational forces.
Ex.: the force exerted by a spring depends on how much the the spring is stretched or compressed and is a conservative force. |
|
|
|
One of the great historical achievements of Newton's laws was...
|
to derive Keplar's laws of planetary motion.
|
|
|
|
In terms of planetary motion, Newtown explained why...
|
1. planets travel in elliptical paths,
2. planets speed up when they are closest to the sun 3. planets obey the laws of periods concerning the time it takes for a planet to make one revolution. |
|
|
|
In terms of solving problems involving force and motion, you must calculate all...
|
3 variables
|
|
|
|
Problems involving inclined planes require considering the ______ and ______________.
|
the vector of force
and calculating the components of the force perpendicular to the surface of the plane and parallel to it. |
|
|
|
Examples of motion in two dimensions
|
1. projectile motion at the surface of the Earth. There's vertical and horizontal motion to consider, which can be solved separately and combined.
2. inclined planes; required considering the vector nature of force and calculating the components of the force perpendicular to the surface of the plane and parallel to it. 3. solving the motion of an object not near the surface of the Earth. 4. the problem of circular motion |
|
|
|
An object near the Earth's surface moves in a...
|
parabola
|
|
|
|
Solving the motion of an object not near the surface of Earth means taking into consideration the...
|
Universal Law of Gravity
|
|
|
|
The problem of circular motion. Explain and give an example.
|
When a force is applied to a moving mass in a direction that is perpendicular to the motion, the object changes direction. The change in direction constitutes an acceleration which is called a centripetal acceleration.
An example, when an automobile turns a corner. |
|
|
|
Centripetal motion
|
When a force is applied to a moving mass in a direction that is perpendicular to the motion, the object changes direction. The change in direction constitutes an acceleration, which is called a centripetal acceleration.
|
|
|
|
A classic example of the conservation of mechanical energy is...
|
the pendulum
|
|
|
|
Describe the conservation of mechanical energy that occurs in a swinging pendulum:
|
The falling motion of the bob is accompanied by an increase in speed.
As the bob loses height and potential energy, it gains speed and kinetic energy. The sum of potential energy and kinetic energy remains constant; The total of the two forms of mechanical energy is conserved. |
|
|
|
A pendulum is considered a case of...
|
simple harmonic motion
|
|
|
|
Harmonic motion occurs...
|
when the force is proportional to the displacement.
While the gravitational force on the pendulum's bob is constant, what is relevant is the component of the force in the direction of motion. This component is proportional to the displacement. |
|
|
|
Hooke's Law
|
In springs, when the component of force in the direction of motion is proportional to the displacement.
F(elastic) = kx |
|
|
|
a mechanical wave is...
|
a disturbance that propagates through a medium at a speed characteristic of the medium.
There is a transfer of energy, there is not a bulk transfer of matter. ex. tuning fork |
|
|
|
the speed of a wave is...
|
the speed the disturbance propagates through the medium.
|
|
|
|
the period of a wave is...
|
the time between disturbances
|
|
|
|
the frequency of a wave is...
|
the inverse of the period
|
|
|
|
the unit measure for frequencies is...
|
the hertz, which is 1/second
|
|
|
|
the amplitude of a wave is...
|
a measure of how much the medium is being distorted.
In the case of water waves, the amplitude is the height of the wave. |
|
|
|
the wavelength of a wave is...
|
the distance between the pulses or individual disturbances.
|
|
|
|
in transverse waves, the...
|
disturbance of the medium is perpendicular to the direction of motion of the disturbance.
ex: water waves |
|
|
|
in longitudinal waves, the...
|
disturbance is parallel to the direction of motion.
ex.: sound |
|
|
|
a tuning fork creates...
|
alternate areas of compression and rarefaction of the air.
|
|
|
|
In the case of sound waves, frequency produces the sensation of...
|
pitch
|
|
|
|
In the case of sound waves, amplitude produces the sensation of...
|
loudness
|
|
|
|
The pitch used for tuning in Western music is...
|
440 hz
|
|
|
|
Visible light, radio waves, x-rays, microwaves, gamma rays, and radar consist of...
|
vibrating electric and magnetic fields.
|
|
|
|
Visible light, radio waves, x-rays, microwaves, gamma rays, and radar are _____ but not _______, because they ____________.
|
waves, but not mechanical waves, because they travel through a vacuum.
|
|
|
|
The speed of radiation is...
|
186,000 miles per second or 3x10⁸ meters per second
|
|
|
|
Who derived the speed of light and when and how?
|
Clerk Maxwell, 19th century, from measurements in electricity and magnetism.
|
|
|
|
How are electric and magnetic fields situated from one another? Or, in other words, they are _____
|
they are perpendicular to each other and to the direction of propagation of the wave
or, in other words, they are transverse |
|
|
|
Electromagnetic radiation is a ______ wave.
|
transverse
|
|
|
|
AM radio waves have a wavelength and frequency of what?
|
wavelength: 100 meters
frequency: 10⁶ Hz |
|
|
|
Gamma rays have a frequency of and what kind of corresponding wavelength?
|
frequency: 10⁹⁺⁹⁺⁶
wavelength: very small |
|
|
|
Seismic waves are _____ waves
|
elastic
|
|
|
|
What are the 2 types of seismic waves?
|
Primary & secondary
|
|
|
|
Primary waves are _____ waves, and are the ______ traveling.
|
longitudinal, fastest
|
|
|
|
Secondary waves are _____ waves, and are _______ traveling.
|
transverse, slower (than P-waves)
|
|
|
|
S waves do not travel through...
|
liquids.
|
|
|
|
The most common cause for water waves is....
|
wind
|
|
|
|
What is fetch?
|
the distance the wind blows
|
|
|
|
What determines how big wave ripples will become?
|
The strength, fetch, and length of gust (duration).
|
|
|
|
Water waves are divided into several parts, what are they?
|
The crest
The trough or valley Wave length Wave height Wave period |
|
|
|
The crest of a wave is...
|
the highest point on wave
|
|
|
|
The trough or valley of a way is the...
|
lowest point between 2 waves
|
|
|
|
The wavelength of a wave is....
|
the horizontal, either between the crests or troughs, of two consecutive waves.
|
|
|
|
Wave height is...
|
a vertical distance between a wave's crest and the next trough.
|
|
|
|
The wave period can be measured by...
|
picking a stationary point and counting the seconds it takes for two consecutive crests or troughs to pass it.
|
|
|
|
If you were to follow a single drop of water during a passing wave, what would you see?
|
you would see it move in a vertical circle, returning to a point near its original position at the wave's end.
These vertical circles are more obvious at the surface. As depth, increases, their effects slowly decrease until completely disappearing about half a wavelength below the surface. |
|
|
|
Superposition means...
|
that two or more different waves can be in the same medium andn at the same time.
|
|
|
|
In constructive interference....
|
the amplitudes of the different waves reinforce one another and the resulting wave has a greater amplitude that the superposed waves.
|
|
|
|
In destructive interference,
|
the amplitudes cancel out to a certain degree.
|
|
|
|
In the case of sound waves, an example of interference is the phenomena of...
|
beats
|
|
|
|
If you superpose two sounds with frequencies that are close in value (e.g. by 10Hz), what happens?
|
There will be a variation in loudness that depends on the difference between the two frequencies.
|
|
|
|
A phenomena common to all waves is the...
|
doppler effect
|
|
|
|
In the doppler effect...
|
if the source of the wave is moving towards the observer, the wavelength is shorter than it would be if the source was stationary.
Likewise, if the source is moving away from the observer, the wavelength is longer. |
|
|
|
The Doppler effect is used to measure...by...
|
the speed of moving objects, by pulsing radar, with a definite wavelength and frequency, at the object. When the pulses hit the object they are reflected. the frequency of the reflected pulses is measured.
|
|
|
|
When a wave is incident upon a boundary between two different media (e.g. light in a vacuum incident upon glass), what happens?
|
Part of the wave is reflected and part of the wave is refracted.
|
|
|
|
When a wave is incident upon a boundary between two different media, the refracted wave ______
|
continues to propagate in the new medium.
|
|
|
|
When a wave is incident upon a boundary between two different media, when is a refracted wave greater?
|
the less the difference that exists between the two media is, the greater the amount of refracted wave.
|
|
|
|
When sound traveling in air hits a brick wall, the....
|
intensity of the reflected sound will be much greater than the intensity of the sound wave propagated inside the wall.
|
|
|
|
The intensity of a wave is...
|
a measure of how much energy is being transported by the wave.
|
|
|
|
Diffraction refers to...
|
the fact that waves have a tendency to spread out in a medium.
|
|
|
|
In diffraction, waves have a tendency to spread out in a medium. Why? What is an implication of this process?
|
Because a wave is a disturbance in a medium and each point of disturbance is a source of further disturbances.
This means waves can travel around barriers placed in the medium. |
|
|
|
The wavelength of light can be measured by...
|
combining the phenomena of diffraction and interference.
|
|
|
|
Because of diffraction, when light is incident upon a double slit, what happens?
|
Each slit will become a new source of light.
|
|
|
|
When light is incident upon a double slit, the two beams will _____ and ______.
|
superimpose and interfere
|
|
|
|
When light is incident upon a double slit, each slit will become a new source of light, and there will be ______ and _____ interference, which will...that can be...
|
constructive & destructive;
will produce dark and bright lines on a screen. the distance between the dark lines, the wavelength of the incident light can be measured. |
|
|
|
Because light has such a small wavelength, it can be assumed...
|
light consists of rays traveling in a straight line
|
|
|
|
Snell's law describes...
|
the phenomena of how light does not change direction if a ray hits the surface of a glass perpendicularly. When it hits glass at an angle there is a reflected ray and a refracted ray.
|
|
|
|
A refracted ray changes direction, when incident upon a smooth glass, because...
|
the speed of light in a glass is less than the speed of light in a vacuum.
|
|
|
|
Wave-particle duality is the...
|
exhibition of both wavelike and particle-like properties by photons (particles of light), electrons, protons, and neutrons.
|
|
|
|
All objects exhibit wave-particle duality to some extent, but for ______ objects the...
|
macroscopic objects, the quantum mechanical wavelength is so small it can't be observed.
|
|
|
|
The existence of shells and energy levels in atoms can be understood from the phenomena of __________.
|
Standing waves
|
|
|
|
Standing waves occur from...
|
the interference of waves traveling in opposite directions but having the same frequency and wavelength.
|
|
|
|
In standing waves, there's no ________, but _______ creates ____ and _____.
|
net propagation of energy, but interference creates nodes and anti-nodes.
|
|
|
|
Shells are created in atoms because...
|
the wavelength of the electron has to be some multiple of the orbit size.
|
|
|
|
In the lowest energy level, the wavelength of the electron is...
|
exactly equal to the circumference of the orbit.
|
|
|
|
The particle-lie property of electromagnetic radiation was discovered with the...
|
photoelectric effect
|
|
|
|
In the photoelectric effect...
|
light striking a metal surface causes electrons to be emitted from the metal.
|
|
|
|
A photon is a particle of light that can have ____ and _____ but without ______.
|
momentum and energy but without mass.
|
|
|
|
For photons/electromagnetic radiation, radio waves behave more like ______ than _____, and gamma rays behave more like _____ than ______.
|
waves than particles
particles than waves. |
|
|
|
Electrostatics is the study of....
|
the study of electric charges
|
|
|
|
An electroscope is...
|
a simple device used to indicate the existence of a positive or negative charge.
|
|
|
|
Describe a simple (and common) electroscope.
|
it's made up of a metal know with very lightweight leaves of aluminum foil attached to it.
When a charged object touches the knob, the leaves push away from each other because like charges repel. |
|
|
|
Grounding is the...
|
removal of static electricity by conduction.
|
|
|
|
A metal rod can be given a charge by placing it in _____.
|
water
|
|
|
|
The concentration of charge is:
|
voltage
|
|
|
|
Describe what happens when you put a metal rod in water.
|
Water molecules are polar. Metal consists of a lattice of positively charged nuclei in a sea of electrons. Water molecules surround a metallic nuclei and cause it to go into solution, leaving behind a negative charge on the metal rod.
|
|
|
|
Voltage is measured in units called:
|
volts
|
|
|
|
In a battery, voltage difference causes...
|
electrons to flow at a slow drift velocity from one terminal to another.
at the same time, metal ions will reattach to one terminal and be removed from the other until the battery goes dead. |
|
|
|
The unit of measurement for current is:
|
ampere
|
|
|
|
Magnets exert force on ______ and on _______.
|
other magnets, and on electric charges.
|
|
|
|
A magnet creates a ______ in the space around it, and it is this that_______.
|
magnetic field; exerts force.
|
|
|
|
Magnetic fields are created by...
|
moving charges
|
|
|
|
A current flowing in a straight wire produces a....
|
a circular magnetic field pointing in a direction determined by the direction of current flow.
|
|
|
|
A current flowing in a straight wire produces a circular magnetic field that has what kind of polarity?
|
none
|
|
|
|
A current flowing through a wire formed into a loop or circle, what happens? (2 points)
|
the magnetic field lines will wenter one side of the loop and exit the other side.
The side they exit is the north pole, and the side they enter is the south pole. |
|
|
|
What can you do with a wire to make a strong magnet. What kind of magnet is this?
|
By wrapping wire around a pole, you can stack current loops
electromagnet |
|
|
|
______ behave like tiny magnets.
|
Electrons
|
|
|
|
Electrons behave like tiny magnets because....
|
they are rotating about their own axis.
|
|
|
|
Besides creating magnetic fields by rotating about their own axis, electrons also magnetic fields by...
|
rotating around the nuclueus
|
|
|
|
Other than electrons, what other subatomic particle produces a magnetic field?
|
proton
|
|
|
|
Most substances are not magnetized because...
|
the magnetic fields produced by electrons, atoms, and protons cancel out.
|
|
|
|
A few substances are ___-magnetic, but all other substances are ___-magnetic.
|
diamagnetic; paramagnetic.
|
|
|
|
With paramagnetic substances...
|
a north pole induces a south pole and the paramagnet is attracted to the magnet.
|
|
|
|
When an object is brought close to a magnet, the object will become magnetized, in other words,.....
|
a north pole and south pole will be induced on the object.
|
|
|
|
Ferromagnetism is a special case of...
|
paramagnetism
|
|
|
|
The main ferromagnetic materials are...
|
iron, nickel, and cobalt.
|
|
|
|
Ferromagnetic materials are connected in such a way that... (2 points)
|
small magnetic domains are created where the magnetic poles of the atoms are aligned.
The north and south poles of the domains are random within the substance, but an external magnetic field will line them up and a permanent magnet can be created. |
|
|
|
How does a compass work? (3 ponts)
|
The Earth produces a magnetic field.
The field is directed away from the geographic south pole, circles the globe, and enters the geographic north pole. The magnet in a compass lines up with the Earth's magnetic field. |
|
|
|
Explain how telegraphs work?
|
electromagnets; when a telegraph key is pushed, current flows through a circuit, turning on an electromagnet which attracts an iron bar. The iron bar hits a sounding bar that responds with a click.
|
|
|
|
An electric motor uses a(n) ______ to change electric energy into mechanical energy.
|
electromagnet
|
|
|
|
Dynamics is the study of...
|
forces and how forces produce motion.
|
|
|
|
Kinematics is the study of...
|
motion without regard to the cause of motion.
|
|
|
|
Objects near the surface of Earth accelerate at:
|
9.8 m/s2.
|
|
|
|
As a falling objects moves closer to the center of the Earth, it's...
|
acceleration increases slightly since the force of gravity increases slightly.
|
|
|
|
Put simply, the law of conservation of energy is that... (2 points)
|
energy never disappears
it just transforms from one form to another. |
|
|
|
Work is performed whenever...
|
a force acts through a distance.
|
|
|
|
Energy is defined as...
|
the ability to do work.
|
|
|
|
Kinetic energy is...
|
the energy of motion
|
|
|
|
An object located on the top of a building has ________ energy because it will acquire _____ energy if it falls.
|
gravitational potential; kinetic
|
|
|
|
In general, mechanical potential energy is _____ or _____ energy.
|
stored or future
|
|
|
|
A coiled spring has _____ energy.
|
elastic
|
|
|
|
Chemical energy is the energy....and can be...
|
stored in atoms and molecules
and can be transformed into heat energy by various chemical reactions. |
|
|
|
Nuclear energy is stored in... and is released....
|
the nuclei of atoms; in nuclear reactors in the form of heat and radiation.
|
|
|
|
The kinetic theory states that...
|
matter consists of molecules in continual random motion.
|
|
|
|
The state of matter depends on...
|
the amount of kinetic energy the molecules possess.
|
|
|
|
It takes ____ to change the phase of a substance.
|
energy
|
|
|
|
The melting point of water is 0 Celsius, at which point will ice convert to water. During this phase change, at what temperature will be the ice & liquid water? Why?
|
0 Celsius. Because the heat energy is being used to change the phase of the water.
|
|
|
|
Heat is called thermal energy to distinguish it from....
|
mechanical energy
|
|
|
|
Another form of thermal energy is _____ energy.
|
internal energy
|
|
|
|
Internal energy is...
|
the mechanical energy possessed by the atoms and molecules in an object.
When we add heat to an object, its internal energy increases. |
|
|
|
When hot and cold items contact each other, what happens?
|
Thermal equilibrium; heat will flow from the hot object to the cold object until the temperatures are the same.
|
|
|
|
The Kelvin scale is based on measurements of...
|
the temperatures of gases and their volumes.
|
|
|
|
If you add one calorie of heat to gram of, for example, lead, the temperature will increase _____.
|
32 degrees celsius
|
|
|
|
Liquid water has twice the _____ heat of _____?
|
specific; ice
|
|
|
|
Conduction occurs when...
|
2 objects are in thermal contact and heat from the hotter object flows into the cooler object.
|
|
|
|
Convection occurs when...
|
heat is transported by the movement of heated substance.
|
|
|
|
A hot object emits... (2 words)
|
infrared radiation
|
|
|
|
How does a thermos bottle or Dewar flask work?
|
The double walls that make up the containers sandwich a vacuum, which greatly lessens thermal equilibrium.
|
|
|
|
Internal energy and heat are forms of energy because they have the ability to...
|
do work (= force times distance)
|
|
|
|
The mechanical equivalent of heat is:
|
4.186 joules = 1 calorie
|
|
|
|
If you stir up a quantity of water until the temperature rises by 1 degree celsius, you will find that it required how much energy?
|
4.186 joules of mechanical energy
|
|
|
|
Mechanical energy is not conserved because...
|
of the force of friction
|
|
|
|
The first law of thermodynamics states...
|
that mechanical energy and internal energy are conserved.
Or, in other words, the increase of the internal energy of a system is equal to the heat added to the system minus the work done by the system. |
|
|
|
The law of conservation of energy is frequently stated to be:
|
Energy can be transformed, but it can neither be created nor destroyed.
|
|
|
|
The zeroth law of thermodynamics states, in effect, , that:
|
you can measure the temperature of a substance with a thermometer
|
|
|
|
The third law of thermodynamics is:
|
that a temperature ob absolute zero can never be reached.
|
|
|
|
The second law of thermodynamics can be formulated a number of different ways:
|
1. heat cannot flow from a colder to a hotter object. Heat always flows from the hotter to the colder object unil the temperatures are the same.
2. A gas will always occupy the entire vessell containing it in a uniform way. There are no parts of the vessel where the density is zero or very low. 3. The amount of disorder in a system is called entropy: the entropy of a closed system always increases. 4. No machine can be imagined that converts heat energy to work energy with 100% efficiency. |
|
|
|
Describe getting out of bed in terms of energy, force, and work.
|
We need to exert our muscle-force over a distance to get out of bed in the morning and we can only do this if we have enough energy.
|
|
|
|
Equation: the of conservation of momentum
|
F=ma
|
|
|
|
What is the definition of energy?
|
there is no definition; there are only definitions of particular kinds of energy.
|
|
|
|
Whenever physicists have discovered energy not being conserved, they have been able to:
|
define a new energy that would save the principle.
|
|
|
|
Equation: potential energy in terms of the height of a pendulum bob
|
PE = (mass)(gravity)(height)
|
|
|
|
What kind of force is gravity? Why?
|
Conservative; because a potential energy can be defined.
|
|
|
|
What are the conservative forces?
|
gravity
electrical magnetic elastic |
|
|
|
The sum of kinetic and potential energy is a...
|
constant
|
|
|
|
The first law of thermodynamics is an example of inventing a new energy called...
|
internal energy
|
|
|
|
Internal energy explains what happens to what and when, and why?
|
mechanical energy when it disappears, because it's transformed into the internal energy of objects exerting the forces that produce mechanical energy.
|
|
|
|
The photoelectric effect refers to:
|
the phenomena of light causing electrons to be ejected from a metal.
|
|
|
|
In terms of the photoelectric effect, energy is conserved if you assume:
|
light is composed of photons and each photon has the energy expressed in terms of Plank's constant and the frequency of the light.
|
|
|
|
Equation: Plank's constant
|
E = hf
|
|
|
|
The form of energy associated with mass:
|
E=mc(squared)
|
|
|
|
Explain the conversion between mass and energy
|
1. the forces binding neutrons and protons together are called nuclear forces or strong forces. The potential energy associated with these forces is called nuclear binding energy.
When neutrons and protons become bound in a nucleus, the mass decreases but there is an increase in the binding energy. Also, protons are not the only positively charged particles. There are positrons, which have the same mass as an electron. In positron-electron annihilation, the mass of the particles is converted into the energy of a photon. |
|
|
|
The most common nuclear reaction is:
|
radioactive decay
|
|
|
|
Radioactive decay occurs when...
|
a nucleus is unstable
|
|
|
|
There are no stable nuclides with atomic numbers greater than:
|
83 (bismuth); also, many isotopes with smaller atomic numbers are unstable.
|
|
|
|
Nuclides decay by emitting:
|
alpha particles,
beat particles positrons, and gamma rays |
|
|
|
What's an alpha particle?
|
Nucleus of a helium atom
|
|
|
|
What's a beat particle?
|
Electrons
|
|
|
|
What's a positron?
|
Elementary particle with the same mass as an electron, but with a positive charge.
|
|
|
|
What are gamma rays?
|
photons
|
|
|
|
Besided emitting all sorts of particles, how else do nuclides decay?
|
by capturing an electron from one of the inner shells. The new nuclide created by the decay may also be unstable.
|
|
|
|
Nuclei decay at different...
|
rates
|
|
|
|
An important quantity in nuclear physics is the binding energy per...
|
nucleon
|
|
|
|
The binding energy of a nucleus is the...
|
sum of the masses of the neutrons and protons minus the mass of the nucleus.
|
|
|
|
In terms of binding energy, there is a ______ in ______ because...
|
a decrease in mass, because the mass is converted into binding energy according to E=mc(squar'd)
|
|
|
|
The nuclei with the greatest binding energy per nucleon is ____; which is why....
|
helium; which is why alpha particles are frequently emitted when nuclei decay.
|
|
|
|
What causes the lack of stability in bigger nuclides?
|
the decrease in binding energy per nucleon
|
|
|
|
Describe how nuclear fission power.
|
Uranium-235 splits into krypton-92 and barium-141 plus three neutrons when it absorbs a neutron.
If there is enough fissionable uranium, a chain reaction can occur. In a nuclear power plant, the chain reaction is controlled with chromium and carbon rods. The fragments have a tremendous amount of kinetic energy, which is used to heat up water for steam turbines. |
|
|
|
Nuclear fusion occurs where?
|
In the sun and in hydrogen bombs, when hydrogen isotopes combine to form helium.
|
|
|
|
In both fission and fusion reactions, there is a ______ in binding energy and a corresponding ____________.
|
decrease; release of energy
|
|
|
|
Radioactive nuclitides are used for what?
|
treatment of cancer cells. The radiation kills both cancer and healthy cells. The healthy cells are better able to repair themselves.
|
|
|
|
Visible light is part of the _________ spectrum, and consists of _______.
|
electrostatic; photons
|
|
|
|
Photons have both ______ and ______ properties.
|
wave and particle
|
|
|
|
Give a non-standard description of wavelength sizes with respect to the following radiation types:
- radio waves - microwaves - infrared radiation - visible light - ultraviolet radiation - X-rays - gamma rays |
football field : radio wave
insects : microwaves point of a needle : infrared radiation protozoa : visible light molecule : ultraviolet radiation atoms : x-rays nuclei : gamma rays |
|
|
|
Visible light and other electromagnetic radiation can be _______ because the...
|
polarized; because the electric and magnetic fields are perpendicular to the direction of the motion of the wave.
|
|
|
|
Polarized light has vibrations confined to....
|
a single plane
|
|
|
|
How do Polaroid sheets work?
|
the block all vibrations except those in a single plane.
Polaroid sheets are made up of long molecules that are aligned in one direction. Only light waves parallel to the molecules passes through. |
|
|
|
Fiber optics works because...
|
light inside the fibers are internally refracted so that it stays inside the fiber until it reaches its destination on the other end.
|
|
|
|
Total internal refraction happens when...
|
a ray of light is incident upon a surface, and the angle of incidence is too small, there will be no refracted ray.
|
|
|
|
How is a convex lens shaped?
|
It's thicker in the middle than at the edges
|
|
|
|
Convex lens causes...
|
parallel beams of light to converge at a point called the focal point.
|
|
|
|
A convex lens can create what between its focal point and itself.
|
small inverted images of objects
|
|
|
|
1 pound = ? newtons
|
4.4 newtons
|
|
|
|
Power is defined by what equation?
|
P = WIt
|
|
|
|
What is the rate at which work is done?
|
power
|
|
|
|
A watt is 1 ? per second
|
joule
|
|
|
|
a horse power is ? (number) ? (unit) per second?
|
550 foot-pounds
|
|
|
|
A lever enables a human to lift heavy things by...
|
transferring a small force exerted for a big distance to a large force exerted for a small distance.
|
|
|
|
When is the efficiency of a simple machine at 100%?
|
when there's no friction
|
|
|
|
The mechanical advantage is the...
|
ratio of the input force to the output force or the output distance to the input distance.
|
|
|
|
Friction is defined as...
|
the work output divided by the work input.
The more efficient a system is, the less energy that is lost within that system. |
|
|
|
Chemical reactions are...
|
the interactions of substances resulting in the chemical change of the substances.
|
|
|
|
Chemical reations involve the _____ and ______ of chemical bonds.
|
breaking and forming
|
|
|
|
Reactants are the...
|
original substances that interact to form the resulting products.
|
|
|
|
Endothermic chemical reactions require the...
|
input of energy
|
|
|
|
Exothermic chemical reactions ______ energy with ______ formation.
|
release energy with product formation
|
|
|
|
______ is conserved in chemical reactions, because....
|
Mass; because the energies of the chemical bonds are so small that the change in mass is negligible.
|
|
|
|
Nuclear (or, atomic) reactions are....
|
reactions that change the composition or structure of atomic nuclei.
|
|
|
|
Nuclear reactions change the number of ...
|
protons and neutrons in the nucleus.
|
|
|
|
The two main types of nuclear reactions are...
|
fission (splitting of nuclei)
fusion (joining of nuclei) |
|
|
|
In nuclear reactions, the ______ energies are so _____ that...
|
binding energies are so great that the change in masses is measurable
|
|
|
|
The basic unit of charge in electricity in the SI system of units is the...
|
ampere
|
|
|
|
When two parallel wires, 1 meter long 1 meter apart, have currents of 1 amp flowing through each of them, the force between the wires will be...
|
2 x 10^-7 newtons
|
|
|
|
Why is there force between parallel electrical lines?
|
The force arises because a current carrying wire produces circular magnetic fields and magnetic fields exert a force on a current carrying wire.
|
|
|
|
A coulomb is defined as...
|
the amount of charge transported by 1 amp of current in one second.
1 coulomb = 1 amp x 1 second |
|
|
|
What did the Millikan oil-drop experiment determine, and how?
|
The charge on an electron was -1.6x10^-19 coulombs.
The charge was measured by measuring the acceleration of tiny oil drops that picked up extra electrons in a electric field. |
|
|
|
Describe what's happening in a battery at its terminals
|
The positive terminal will have a lower density of electrons on it than the negative terminal. This means the potential energy of the electrons on one terminal will be greater than the potential energy of the electrons on the other terminal.
|
|
|
|
In terms of a better, potential is a property of ______ in the vicinity of charges.
|
space
|
|
|
|
The potential energy of a charge is measured in...
|
volts
|
|
|
|
Volts is a unit equal to:
|
one joule divided by one coulomb
|
|
|
|
The two terminals on a battery produce a potential ______ (represented by what unit?)
|
difference (V)
|
|
|
|
When a wire is connected to the two terminals of a battery, a current will flow in the wires because...
|
the potential difference produces an electric field in the wire and the electric field exerts a force on the electrons, which are free to move in a conductor.
|
|
|
|
Electrons in a conductor move very _______, because...
|
slowly (1 to 2 centimeters per hour); because, they bump into the positively charged nuclei of the metal.
|
|
|
|
If you double the length of a wire, the values of electric field, electron speed, and current will be reduced by ______?
|
half
|
|
|
|
If you double the diameter of a electric wire, the current will ... Why?
|
increase by a factor of four; because there will be four times as many electrons drifting through the wire.
|
|
|
|
The resistance of a wire is the...
|
ratio of the voltage difference defined by Ohm's law.
|
|
|
|
An ohm is defined as...
|
1 volt divided by 1 amp
|
|
|
|
Electricity can change the _________ of a material.
|
chemical composition
|
|
|
|
Resistors are used to...
|
regulate volume on electronic devices, or to dim lights in a dimmer switch.
|
|
|
|
Ohm's law states:
(equation) |
V= I x R
V= voltage difference I = current R= resistance |
|
|
|
Resistance is measured in:
|
Ohms
|
|
|
|
A material through which electrical chargers do not move easily:
|
insulator
|
|
|
|
an electric current is...
|
a path along which electrons flow
|
|
|
|
A series circuit is...
|
one where the electrons have only one path along which they can move.
|
|
|
|
A parallel circuit is...
|
one where the electrons have more than one path to move along.
|
|
|
|
What does a voltmeter do?
|
measures the potential energy of a point on an electric circuit
|
|
|
|
What does an ammeter do?
|
measure current on a circuit
|
|
|
|
Tectonic plates are...
|
rigid blocks of Earth's crust and upper mantle, and make up the lithosphere.
|
|
|
|
The major plates of the lithosphere are named after what?
|
the continents they are "transporting"
|
|
|
|
Volcanoes, mountain ranges, and earthquake zones are usually located where?
|
at plate boundaries, where the plates interact by spreading apart, pressing together, or sliding past each other
|
|
|
|
Rifting is a process when...
|
boundaries form between spreading plates where the crust is forced apart
|
|
|
|
What happens during subduction? When does this usually happen?
|
a process in which a dense plate collides with a less dense plate, and slides under the lighter one and plunges into the mantle.
|
|
|
|
Subduction happens in the...
|
subduction zone
|
|
|
|
A subduction zone is usually seen ...
|
on the sea-floor as a deep depression called a trench
|
|
|
|
Evidence that supported the theory of plate tectonics include...
|
the fit of continents, fossils, similarities of rock type and rock structure, and Earth magnetism
|
|
|
|
Who advanced the theory of continental drift?
|
Alfred Wegener
|
|
|
|
Who suggested that 200 million years ago there was a supercontinent called Pangaea?
|
Alfred Wegener
|
|
|
|
The theory of continental drift and Pangaea were advanced by who and when?
|
Alfred Wegener, 1915
|
|
|
|
What fossil evidence supports plate tectonic theory, and why?
|
the mesosaur fossil, which is found only in eastern South America and Southern Africa.
|
|
|
|
What geological evidence supports a supercontinent?
|
Rocks in eastern Brazil and rocks found in northwestern Africa.
The Appalachians in the U.S. and mountain ranges in Europe. |
|
|
|
Orogeny is the term given to...
|
natural mountain building
|
|
|
|
The physical composition of mountains include what rock types?
|
- igneous
- metamorphic - sedimentary |
|
|
|
Name the different types of mountains.
|
- Folded
- Fault-block - Dome - Upwarped - Volcanism |
|
|
|
What are folded mountains? Give real examples.
|
they are produced by the folding of rock layers during their formation. This type includes the highest of mountains.
Examples: Alps, Himalayas |
|
|
|
What are fault-block mountains? Give real examples.
|
Are created when plate movement produces tension forces instead of compression forces. The area under tension produces normal faults and rock along these faults is displaced upwards.
Examples: Utah, Arizona, & New Mexico) |
|
|
|
What are dome mountains?
|
Are formed as magma tries to push up through the crust but fails to break the surface. Dome mountains resemble a huge blister on the Earth's surface.
|
|
|
|
What are upwarped mountains? Give real examples.
|
Are created in association with a broad arching of the crust. They can also be formed by rock thrust upward along high angle faults.
Examples: Black hills of South Dakota |
|
|
|
What's Volcanism, and it's relation to mountains?
|
It's a term given to the movement of magma through the crust and its emergence as lava onto the Earth's surface.
Volcanic mountains are built up by successive deposits of volcanic materials. |
|
|
|
Types of Volcanic Mountains:
|
- Shield
- Cinder cone - Composite |
|
|
|
What are Shield Volcanoes?
|
Are associated with quiet eruptions.
Repeated lava flow builds this type of volcano into the largest volcanic mountain. Mauna Loa found in Hawaii, is the largest volcano on Earth. |
|
|
|
What are Cinder Cone volcanoes?
|
Are associated with explosive eruptions as lava is hurled high into the air in a spray of droplets of various sizes. The droplets cool and harden into cinders and particles of ash.
The cinder and ash settle around the vent, forming small but quite rapid, volcanoes. |
|
|
|
What are composite volcanoes?
|
Described as being built by both lava flows and layers of ash and cinder.
Examples: Mt. Fuji; Mt. Saint Helens; Mt. Vesuvius. |
|
|
|
Mechanisms of producing mountains:
|
- Folding
- dip-slip fault - Reverse faults - strike-slip fault - transform fault - oblique-slip fault - cooling lava - intrusive rock - extrusive rock - Dikes - Caldera |
|
|
|
Folded mountains are produced by...
|
the folding of rock layers. Crustal movements may press horizontal layers of sedimentary rock together from the sides, squeezing them into wavelike folds.
|
|
|
|
In terms of folded mountains, anticlines refer to...
|
up-folded sections of rock
|
|
|
|
In terms of folded mountains, synclines refer to...
|
down-folded sections of rock
|
|
|
|
Faulting movement can be ______, ________, or _______.
|
horizontal, vertical or oblique
|
|
|
|
Faults are fractures in the Earth's crust which have been created by...
|
either tension or compression forces transmitted through the crust.
|
|
|
|
Faultings are categorized on the basis of...
|
the relative movement between the blocks on both sides of the fault plane.
|
|
|
|
A dip-slip fault occurs when...
|
the movement of the plates is vertical and opposite.
|
|
|
|
In a dip-slip fault, the displacement is in the direction of the...
|
inclination, or dip, of the fault.
|
|
|
|
Dip-slip faults are classified as _______ faults, when....
|
normal; when the rock above the fault plane moves down relative to the rock below
|
|
|
|
Reverse faults are created when...
|
rock above the fault plane moves up relative to the rock below.
|
|
|
|
When are reverse faults referred to as thrust faults?
|
when reverse faults have a very low angle to the horizontal
|
|
|
|
What are strike-slip faults?
|
Are faults in which the dominant displacement is horizontal movement along the trend or strike (length) of the fault
|
|
|
|
What's a transform fault?
|
When a large strike-slip fault is associated with plate boundaries
|
|
|
|
What's a well known transform fault in the U.S.?
|
The San Andreas Fault in California
|
|
|
|
Describe a oblique-slip fault
|
Faults that have both vertical and horizontal movement
|
|
|
|
Igneous rock is formed when...
|
when lava cools
|
|
|
|
Intrusive rock includes...
|
any igneous rock that was formed below the Earth's surface.
|
|
|
|
Batholiths are...
|
the largest structures of intrusive-type rock and are composed of near granite materials
|
|
|
|
Batholiths are the core of what U.S. geographical feature?
|
the Sierra Nevada Mountains
|
|
|
|
Extrusive rock includes...
|
any igneous rock that was formed at the Earth's surface
|
|
|
|
What's the volcano neck?
|
where in an inactive volcano that has magma solidified in its pipe.
|
|
|
|
Why might the volcano neck be the only evidence of the past presence of an active volcano?
|
Because it's resistant to erosion
|
|
|
|
During the most recent ice age, a large part of North America was covered by a...
|
continental glacier
|
|
|
|
Evidence of a continental glaciation includes... (4)
|
- abrasive grooves
- large boulders from northern environments dropped in southernly locations - glacial troughs created by the rounding out of steep valleys by glacial scouring - presence of cirques |
|
|
|
Glacial cirques are...
|
is an amphitheatre-like valley head, formed at the head of a valley glacier by erosion
|
|
|
|
A moraine is...
|
any glacially formed accumulation of unconsolidated glacial debris (soil and rock) which can occur in currently glaciated and formerly glaciated
|
|
|
|
What evidence helps support the theory of periods of warmth during the past ice ages?
|
remains of plants and animals found in warm climate that have been discovered in moraines and out wash plains.
|
|
|
|
The Ice Age began about...
|
2-3 million years ago.
|
|
|
|
What are some theories relating to the origin of glacial activity?
|
Plate tectonics: continental masses, now in temperate zones, were at one time blanketed by ice and snow.
Earth's orbit around the Sun: changes in the angle of the Earth's axis, and the wobbling of the Earth's axis; correlated between climatic sensitive microorganisms and the changes in the Earth's orbital status. |
|
|
|
Describe the U.S.'s glacial history
|
About 12,000 years ago, a vast sheet of ice covered a large part of the northern United States. This huge, frozen mass had moved southward from the northern regions of Canada as several large bodies of slow-moving ice, or glaciers.
|
|
|
|
What is an ice age?
|
A time period in which glaciers advance over a large portion of a continent.
|
|
|
|
What is a glacier?
|
Is a large mass of ice that moves or flows over the land in response to gravity. Glaciers form among high mountains and in other cold regions.
|
|
|
|
What are the two types of glaciers?
|
Valley & continental
|
|
|
|
What do valley glaciers do?
|
Erosion by valley glaciers is characteristic of U-shaped erosion. They produce sharp peaked mountains such as the Matterhorn in Switzerland.
|
|
|
|
What do continental glaciers do?
|
They often ride over mountains in their paths leaving smoothed, rounded mountains and ridges.
|
|
|
|
The biological history of the Earth is partitioned into...
|
four major eons.
|
|
|
|
The most recent eon is the ______ (? years), and marks what?
|
Phanerozoic eon (570 million years ago); marks the beginning of life.
|
|
|
|
The four eons are broken into...
|
10 eras
|
|
|
|
The current eon has the following eras: (describe each)
|
Cenozoic (age of recent life)
Mesozoic (age of middle life) Paleozoic (age of ancient life) |
|
|
|
Each era is divided into:
|
periods
|
|
|
|
Cenozoic era is subdivided into: (recall time ranges)
|
Quaternary period (1.6 Ma - present)
Tertiary period (66 - 1.6 Ma) |
|
|
|
Mesozoic era is subdivided into: (recall time ranges)
|
Cretaceous period (144-66 Ma)
Jurassic & Triassic (245-144 Ma) |
|
|
|
Paleozoic era is subdivided into: (recall time ranges)
|
Permian & Carboniferous (360-245 Ma)
Devonian (408-360 Ma) Silurian & Ordovician (505-408 Ma) Cambrian (570-505 Ma) |
|
|
|
The end of an era is most often characterized by... (3 points)
|
1. a general uplifting of the crust
2. the extinction of the dominant plants or animals 3. the appearance of new life-forms. |
|
|
|
Each of the 12 periods is broken up into ______, usually described as _____, ______, and _____.
|
epochs; early, middle, and late.
|
|
|
|
On the ______ epochs have names. They are: (7)
|
Cenozoic
1. Holocene 2. Pleistocene 3. Pleistocene 4. Miocene 5. Oligocene 6. Eocene 7. Paleocene |
|
|
|
Describe the characteristics of this period: Quaternary
|
The Ice Age occurred, and human beings evolved.
|
|
|
|
Describe the characteristics of this period: Tertiary
|
Mammals and birds evolved to replace the great reptiles and dinosaurs that had just become extinct.
Forests gave way to grasslands, and the climate became cooler. |
|
|
|
Describe the characteristics of this period: Cretaceous
|
Reptiles and dinosaurs roamed the Earth.
Most of the modern continents had split away from the large landmass, Pangea, and many were flooded by shallow chalk seas. |
|
|
|
Describe the characteristics of this periods: Jurassic & Triassic
|
Reptiles were beginning to evolve. Pangea started to break up. Deserts gave way to forests and swamps.
|
|
|
|
Describe the characteristics of this periods: Permian & Carboniferous
|
Continents came together to form one big landmass, Pangea.
Forests (that formed today's coal) greww on deltas around the new mountains, and deserts formed. |
|
|
|
Describe the characteristics of this period: Devonian
|
Continents started moving toward each other.
The first land animals, such as insects and amphibians, existed. Many fish swam in the seas. |
|
|
|
Describe the characteristics of this periods: Silurian & Ordovician
|
Sea life flourished, and the first fish evolved.
The earliest land plants began to grow around shorelines and estuaries. |
|
|
|
Describe the characteristics of this period: Cambrian
|
No life on land, but all kinds of sea animals existed.
|
|
|
|
A sequential history of geology can be determined by what methods?
|
by fossil content (principle of fossil succession) of a rock system.
And by stratigraphy |
|
|
|
What is stratigraphy?
|
The process of classifying a rock system by observing its superposition within a range of systems.
Rock systems are arranged in their correct chronological order. |
|
|
|
What's Uniformitarianism?
|
A fundamental concept in modern geology, stating that the physical, chemical, and biological laws that operated in the geological past operate in the same way today.
The forces and processes that we observe presently shaping our planet have been at work for a very long time. |
|
|
|
Catastrophism is the concept that:
|
the Earth was shaped by catastrophic events of a short term nature.
|
|
|
|
Estimates of the Earth's age have been made possible with the discovery of:
|
radiation, and the invention of instruments that can measure the amount of radioactivity in rocks.
|
|
|
|
Absolute dating
|
the use of radioactivity to make accurate determinations of Earth's age
|
|
|
|
Before using radioactivity, a geological time scale was developed using:
|
relative dating and the law of superposition.
|
|
|
|
What are lines of latitude?
|
Imaginary lines drawn east and west of the parallel to the equator.
|
|
|
|
What are meridians?
|
Imaginary lines drawn north and south at right angles to the equator and from pole to pole.
|
|
|
|
Longitude is a term used...
|
to describe distances in degrees east or west of a 0 degree meridian.
|
|
|
|
The prime meridian is the...
|
0 degree meridian and it passes through Greenwich, England.
|
|
|
|
Time zones are determined by...
|
longitudinal lines
|
|
|
|
Each time zone represents...
|
1 hour
|
|
|
|
Each time zone is roughly how wide?
|
15 degrees
|
|
|
|
While time zones are based on ______, they do not strictly follow lines of _______. Time zone boundaries are subject to ________ decisions.
|
meridians; longitude; political
|
|
|
|
A contour lines is...
|
a line on a map representing an imaginary line on the ground that has the same elevation above sea level along its entire path.
|
|
|
|
Contour intervals usually are given in...
|
even numbers or as a multiple of five.
|
|
|
|
Relief describes...
|
how much variation in elevation an area has.
|
|
|
|
Five general rules should be remembered in studying contour lines on a map:
|
1. Contour lines close around hills and basins or depressions.
2. Contours lines never cross. 3. Contour lines appear on both sides of an area where the slope reverses direction. 4. Contour lines form V's that point upstream when they cross streams. 5. All contours lines either close (connect) or extend to the edge of the map. No map is large enough to have all its contour lines to close. |
|
|
|
Hachur lines are used to... and look like....
|
show depressions: short lines placed at right angles to the contour line and they always point toward the lower elevation.
|
|
|
|
A contour line that has hachures is called a...
|
depression contour
|
|
|
|
In terms of slope, contour lines lines show where an imaginary...
|
horizontal plane would slice through a hillside or cut both sides of a valley
|
|
|
|
Topographic maps are also referred to as...
|
quadrangles
|
|
|
|
Maximum relief refers to...
|
the difference in elevation between the high and low points in the area being considered.
|
|
|
|
World weather patterns are greatly influenced by...
|
ocean surface currents in the upper layer of the ocean.
|
|
|
|
Deep-surface currents are...
|
ocean currents that flow deep below the surface.
|
|
|
|
Sub-surface currents are influenced by factors, such as: (2)
|
- locations of landmasses in the current's path
- the Earth's rotation |
|
|
|
Surface currents are caused by _____, and are classified by ______.
|
winds; temperature
|
|
|
|
Cold ocean currents originate in the ______ regions and flow through ______ water that is ______
|
polar regions: surrounding water; measurably warmer.
|
|
|
|
Warm currents are...
|
currents with a higher temperature than the surrounding water
|
|
|
|
Warm currents can be found near the...
|
equator
|
|
|
|
Warm currents follow what kind of routes?
|
swirling routes around the ocean basins and the equator.
|
|
|
|
What are the two main surface currents that flow along the coastlines of the U.S.?
|
The Gulf Stream and the California Current.
|
|
|
|
The Gulf stream is _____ current, and flows from the ______ to _____.
|
warm; equator; to the northern parts of the Atlantic
|
|
|
|
The California Current is a _____ current that originates in the ____ regions and flows...
|
cold; Arctic; southward along the west coast of the U.S.
|
|
|
|
What also creates ocean currents?
|
differences in water density
|
|
|
|
Water tends to flow from a ______ area to a _____ area.
|
denser; less dense
|
|
|
|
Currents that flow because of a difference in the density of the ocean water are called:
|
density currents
|
|
|
|
Besides water density, what else can form a density current?
|
water that has salinity different from the surrounding water
|
|
|
|
Who studied and named the Gulf Stream?
|
Benjamin Franklin
|
|
|
|
Movement of ocean water is caused by...(5)
|
- wind
- the sun's heat energy - the Earth's rotation - the moon's gravitational pull on the Earth - by underwater earthquakes |
|
|
|
Most ocean waves are caused by:
|
wind
|
|
|
|
How does the wind's impact cause waves?
|
wind blowing over the surface of the ocean transfer energy (friction) to the water and causes waves to form.
|
|
|
|
Describe the Florida peninsula.
|
It's a porous plateau of limestone on top of bedrock.
|
|
|
|
Florida's limestone layers is covered with _____ soils, which were deposited over millions of years as _________.
|
sandy; global sea levels rose and fell.
|
|
|
|
During the last glacial period, Florida was a much.... made up of...
|
wider peninsula; grassy plains with few trees
|
|
|
|
The largest wetland in the U.S.
|
Florida's Everglades
|
|
|
|
The Florida Everglade system spans...
|
begins near Orlando with the Kissimmee River, which discharges into the vast but shallow Lake Okeechobee.
|
|
|
|
During the wet season, what happens to the water in Lake Okeechobee?
|
Water leaves the lake, forming a slow-moving river 60 miles wide and over 100 miles long, flowing southward across a limestone shelf to Florida Bay and the southern end of the state.
|
|
|
|
A fossil is...
|
the remains or trace of an ancient organism that has been preserved naturally in the Earth's crust.
|
|
|
|
______ rocks are rich sources of fossil remains.
|
Sedimentary
|
|
|
|
Few fossils are found in ______ rock and virtually none found in ______ rocks.
|
metamorphic; igneous
|
|
|
|
Sedimentary rock are rich sources of fossil remains because...
|
those fossils found in layers of sediment were embedded in the slowly forming sedimentary rock strata.
|
|
|
|
The best-preserved animal remains have been discovered in...
|
natural tar pits
|
|
|
|
Fossil molds are...
|
the hollow spaces in a rock previously occupied by bones or shells.
|
|
|
|
A fossil cast is..
|
a fossil mold that fills with sediments or minerals that later hardens forming a cast.
|
|
|
|
Fossil tracks are...
|
the imprints in hardened mud left behind by birds or animals.
|
|
|
|
What is lithification?
|
the process of when fluid sediments are transformed into solid sedimentary rocks.
|
|
|
|
One very common process affecting sediments is...
|
compaction
|
|
|
|
Compaction is...
|
the process where the weights of overlying materials compress and compact the deeper sediments.
|
|
|
|
The compaction process leads to....
|
cementation
|
|
|
|
Cementation is when...
|
sediments are converted to sedimentary rock.
|
|
|
|
Igneous rocks can be classified according to...
|
- texture
- composition - the way they formed |
|
|
|
Describe who igneous rocks form, that explains texture/composition variances:
|
Molten rock pouring out onto the surface of the earth, is called lava.
As magma cools, the elements and compounds begin to form crystals. The slower the magma cools, the larger the crystals grow. Rocks with large crystals are said to have a course-grained texture. Rocks that cool rapidly before any crystals can form have a glassy texture. |
|
|
|
Granite is a _____ rock type, with a _____ grain.
|
igneous; course
|
|
|
|
Rocks that cool rapidly before any crystals can form have a....
|
glassy texture.
|
|
|
|
An example of glassy rock is:
|
obsidian, or volcanic glass
|
|
|
|
Metamorphic rocks are formed by... (2 points)
|
high temperatures and great pressures
|
|
|
|
The outcome of metamorphic changes (in rocks) include:
|
- deformation by extreme heat and pressure
- compaction - destruction of the original characteristics of the parent rock - bending and folding while in a plastic stage - the emergence of completely new and different minerals due to chemical reactions with heated water and dissolved minerals. |
|
|
|
Metamorphic rocks are classified into 2 groups:
|
Foliated (leaflike) rocks
Unfoliated. |
|
|
|
Foliated rocks consist of...
|
compressed, parallel bands of minerals, which give the rocks a striped appearance.
|
|
|
|
Examples of foliated rocks include:
|
slate, schist, and gneiss
|
|
|
|
Unfoliated rocks are not _____ and examples include:
|
banded; quartzite, marble, and anthracite.
|
|
|
|
Minerals must adhere to five criteria:
|
1. non-living
2. formed in nature 3. solid in form 4. their atoms form a crystalline pattern 5. its chemical composition is fixed within narrow limits. |
|
|
|
The major groups of minerals are: (6)
|
- silicates
- carbonates - oxides - sulfides - sulfates - halides |
|
|
|
The largest mineral group is the: (examples)
|
silicates; silicon, oxygen, and one or more other elements.
|
|
|
|
2 things determine mineral type:
|
chemical composition & crystal structure
|
|
|
|
Polymorphs are:
|
two or more minerals having identical chemical composition, but varied crystal structure.
|
|
|
|
Two common polymorphs that demonstrate the influence of crystal structure on physical properties.
|
Diamonds & Graphite
|
|
|
|
Silicate minerals are composed mostly of:
|
silicon and oxygen
|
|
|
|
Silicate is the _____ class of minerals, and includes:
|
most abundant; quartz, garnets, micas, and feldspars.
|
|
|
|
Carbonate class minerals are formed from compounds including:
|
carbonate ions
|
|
|
|
Carbonate minerals are commonly found in what environments, and why?
|
marine; where minerals can form via dissolution and precipitation
|
|
|
|
Common examples of Carbonates
|
Nitrate and borate
|
|
|
|
Sulfate minerals contain
|
sulfate ions
|
|
|
|
Sulfates are formed near...
|
bodies of water where slow evaporation allows precipitation of sulfates and halides.
|
|
|
|
Examples of sulfates include:
|
gypsum, celestite, barite
|
|
|
|
Halide minerals include all minerals formed from...
|
natural salts
|
|
|
|
Like sulfides, halides are formed in what environments?
|
evaporative settings
|
|
|
|
Examples of halide minerals include:
|
fluorite, halite, and sylvite
|
|
|
|
Oxide minerals contain _____ compounds, including...
|
oxide; iron oxide, magnetite oxide, and chromium oxide
|
|
|
|
Oxide minerals are formed by...
|
various processes including precipitation and oxidation of other minerals.
|
|
|
|
Oxide minerals are important in...
|
mining
|
|
|
|
Examples of oxide minerals include:
|
Hematite, chromite, rutile, and magnetite
|
|
|
|
Sulfide minerals are formed from _____ compounds
|
sulfide
|
|
|
|
What kind of metal ores are found in the Sulfide class?
|
important ones
|
|
|
|
Examples of sulfide minerals include:
|
pyrite (fool's gold) & galena
|
|
|
|
Phosphate minerals include materials containing ____ ions & ....
|
phosphate; any mineral with a tetrahedral molecular geometry in which an element is surrounded by four oxygen atoms.
|
|
|
|
Phosphates are important biologically, as the are common in...
|
teeth and bones
|
|
|
|
Examples of minerals in phosphates include:
|
phosphate, aresenate, vanadate, and antimonte.
|
|
|
|
Element class minerals are formed from ______, whether they are....
|
pure elements; metallic, semi-metallic or non-metallic.
|
|
|
|
Examples of element class minerals include:
|
gold, silver, copper, bismuth, and graphite, as well as natural alloys such as electrum and carbides.
|
|
|
|
Describe frost wedging:
|
is the cycle of daytime thawing and refreezing at night. This cycle causes large rock masses to be broken into small pieces.
|
|
|
|
Physical weathering is:
|
the process by which rocks are broken down into smaller fragments without undergoing any change in chemical composition.
|
|
|
|
Exfoliation is
|
the peeling away of the outer layers from a rock
|
|
|
|
Chemical weather is:
|
the breaking down of rocks through changes in their chemical composition
|
|
|
|
The main agents of chemical weathering:
|
- water
- oxygen - carbon dioxide |
|
|
|
How does water and carbon dioxide weather rocks?
|
They combine chemically, producing a weak acid.
|
|
|
|
Another word for sedimentation is:
|
deposition
|
|
|
|
Soils are composed of particles of:
|
- sand
- clay - various minerals - tiny living organisms - humus - decayed remains of flora/fauna |
|
|
|
Soils are divided into ____ classes according to their _____.
|
three; texture
|
|
|
|
The 3 classes of soils are:
|
- sandy soils
- clay soils - loamy soils |
|
|
|
Sand soils are described as:
|
gritty, and their particles do not bind together firmly.
Sandy soils are porous, do not hold much water |
|
|
|
Clay soils are described as:
|
smooth and greasy; their particles bind together firmly.
Clay soils are moist and usually do not allow water to pass through them easily. |
|
|
|
Loamy soils can be described as:
|
feeling somewhat like velvet and their particles clump together.
Loamy soils are made up of sand, clay, and silt. They hold water but some water can pass through. |
|
|
|
In addition to 3 main classes (what are they?), soils are further grouped into three major types based upon their ________.
|
based on composition
- pedalfers - pedocals - laterites |
|
|
|
Pedalfers form in...
|
the humid, temperate climate of the eastern U.S.
|
|
|
|
Pedalfer soils contain large amounts of.... making the soild look...
|
iron oxide and aluminum-rich clays, making th soil a brown to reddish brown color.
|
|
|
|
Pedalfer soils supports _______ type vegetation.
|
forest
|
|
|
|
Pedocals are found in...
|
the western U.S. where the climate is dry and temperate.
|
|
|
|
Pedocal soils are rich in _______.
|
calcium carbonate
|
|
|
|
Pedocal soils supports what kind of vegetation (2)?
|
grasslands & bush
|
|
|
|
Laterites are found...
|
where the climate is wet and tropical. Large amounts of water flow through this soil.
|
|
|
|
Laterites are what color and rich in what?
|
red-orange; rich in iron and aluminum oxides.
|
|
|
|
What kind of vegetation does laterite soil support?
|
It's not very fertile, containing very little humus.
|
|
|
|
Dry air is composed of three basic components:
|
- dry gas
- water vapor - solid particles (dust from soil,etc.) |
|
|
|
The most abundant dry gases in the atmosphere are: (most to least)
|
- nitrogen
- oxygen - argon - carbon dioxide |
|
|
|
Layers of the atmosphere are: (closest to farthest)
|
- troposphere
- stratosphere - mesosphere - thermosphere - exosphere |
|
|
|
Facts about the troposphere:
|
- closest to Earth's surface
- all weather occurs here - Air temp. decreases w/increasing altitude - 7 miles thick |
|
|
|
Facts about the stratosphere:
|
- contains very little water vapor
- clouds in this layer are extremely rare - ozone is located in the upper part - temperature is fairly constant, but does increase somewhat b/c of the absorption of solar energy & ultraviolet rays. |
|
|
|
Facts about the mesosphere:
|
- air temperature decreases with height
- is the coldest layer (-100 C at the top) |
|
|
|
Facts about the thermosphere:
|
- extends upward into space
- oxygen molecules absorb energy from the sun, causing temperatures to increase with height. - here charged particles or ions and free electrons can be found |
|
|
|
Facts about the exosphere:
|
- gas molecules are very far apart
- includes the Van Allen belts, which are energetic charged particles held together by Earth's magnetic field. |
|
|
|
Clouds form when:
|
air above the surface cools below the point when liquid water forms
|
|
|
|
The shape of a cloud depends on:
|
the air movement that forms it
|
|
|
|
Stratiform clouds are formed by:
|
horizontal air movement
|
|
|
|
Cumuliform clouds are formed by:
|
vertical air movements
|
|
|
|
El Nino refers to:
|
a sequence of changes in the ocean and atmospheric circulation across the Pacific Ocean.
|
|
|
|
Describe the El Nino sequence:
|
The water around the equator is unusually hot every 2 to 7 years.
Trade winds normally blow east to west across the equatorial latitudes, piling warm water in the western Pacific. A huge mass of heavy thunderstorms usually forms in the area and produces vast currents of rising air that displaces heate poleward. This helps create the strong mid-latitude jet streams. The word's climate patterns are disrupted by this change in location of thunderstorm activity. |
|
|
|
Air currents are:
|
masses of air moving toward or away from the Earth's surface.
|
|
|
|
Wind is:
|
air moving parallel to Earth's surface.
|
|
|
|
Weather conditions are generated by:
|
winds and air currents carrying large amounts of heat and moisture from one part of the atmosphere to another.
|
|
|
|
Wind speeds are measured by instruments called:
|
anemometer
|
|
|
|
Wind belts are:
|
in each hemisphere, and consist of convection cells that encircle the Earth.
|
|
|
|
There are three major wind belts:
|
1. trade winds
2. prevailing westerlies 3. polar easterlies |
|
|
|
Wind belt formation depends on:
|
the differences in air pressures that develop in the doldrums, the horse latitudes, and the polar regions.
|
|
|
|
What are the Doldrums?
|
The surround the equator. Within this belt heated air usually rises straight up into the Earth's atmosphere.
|
|
|
|
What are the Horse latitudes?
|
are regions of high barometric pressure with calm and light winds
|
|
|
|
What are the Polar regions, in terms of wind belts?
|
The contain cold dense air that sinks to the Earth's surface.
|
|
|
|
Winds caused by local temperature changes include:
|
sea breezes and land breezes.
|
|
|
|
Sea breezes are caused by:
|
the unequal heating of the land and an adjacent, large body of water.
Land heats up faster than water. The movement of cool ocean air toward the land is a sea breeze. |
|
|
|
A land breeze is:
|
a breeze that blows from the land to the ocean or a large lake.
|
|
|
|
Monsoons are:
|
huge wind systems that cover large geographic areas and that reverse direction seasonally.
|
|
|
|
Relative humidity is:
|
the actual amount of water vapor in a certain volume of air compared to the maximum amount of water vapor this air could hold at a given temperature.
|
|
|
|
The dew point is:
|
the air temperature at which water vapor begins to condense
|
|
|
|
Facts about tropical rain forests:
|
- climatic changes currently limit this biome to about six percent of the Earth's surface.
- found in South America, Africa, New Guinea, Malaysia, Burma, and Indonesia. - 50% of all the species in the world are found in the rain forest |
|
|
|
Facts about deserts:
|
- sand accounts for 15% of desert terrain.
- most deserts are bare rock or pebbles and gravel areas. |
|
|
|
What is a tundra?
|
- vast regions in which the subsoil is permanently frozen.
- climate is extremely cold located in polar regions |
|
|
|
Facts about tundras:
|
- Moss and lichens exist in this fragile environment
- The Indians, Aleuts, and Eskimos had little effect on the tundra's ecosystem. |
|
|
|
What are Taiga?
|
are swampy and coniferous forests.
- The temperature is mild. - is often laced with rivers and streams |
|
|
|
Facts about Taiga:
|
- niche created by the cool shade of the large trees, there are a great variety of plants, including moss, lichens, and ferns.
- soils of the taiga thaw out completely in the summer and are home to lots of invertebrates and vertebrates which break down the organic detritus, enriching the soil. |
|
|
|
What are grasslands?
|
Are called different names: prairies, in North America; steppes, in Asia; savannas, in Africa and Australia.
- grasses have deep root systems. - mild climate - very rich top soil; farming has converted much of the grasslands into farms. - 11% of Earth is arable - the animals of this region tend to be fast and have mottled colors to blend in with the dry grass. |
|
|
|
The impacts of altitude upon climatic conditions are primarily related to: (2)
|
temperature and precipitation
|
|
|
|
As altitude increases, climatic conditions become:
|
increasingly drier and colder
|
|
|
|
As altitude increases solar radiation becomes _________, while the effects of convection forces _________.
|
more severe; minimalized
|
|
|
|
Proximity to land or water masses produces climatic conditions based upon the available ______.
|
moisture
|
|
|
|
Climate becomes _____ and ______ as the distance from the equator increases.
|
drier and colder
|
|
|
|
Sinkholes, caves, and caverns are sometimes formed by dissolved ______.
|
Limestone
|
|
|
|
Groundwater usually contains large amounts of ________, especially if the water flows through ______.
|
dissolved minerals; limestone
|
|
|
|
Stalagmites are made from:
|
deposits of calcium carbonate
|
|
|
|
What is Karst topography?
|
It's a specific type of rock formation with distinctive surface shapes.
|
|
|
|
How are karst features formed?
|
they're formed when mildly acidic (carbonic acid) water dissolves bedrock (such as limestone or dolostone)
|
|
|
|
In terms of Karst topography, how do complex, underground, water drainage systems form?
|
Acidic water dissolves surface rock and causes fractures. These fractures enlarge over time and as large gaps are formed, underground drainage systems develop which allow even more water to flow in and dissolve the rock.
|
|
|
|
Large visible features formed by Karst topography can be found in the U.S., most notably in: (2)
|
Arkansas and Missouri
|
|
|
|
Leeching is the...
|
extraction of substances from a solid by a liquid. Can be good or bad.
|
|
|
|
Buys-Ballot's law states...
|
that wind travels counterclockwise around low pressure zones in the northern hemisphere and clockwise in the southern hemisphere.
This is a consequence of the original observation, that in the northern hemisphere, if you stand with your back to the wind, the low-pressure area will be on your left. |
|
|
|
The water table is:
|
the level at which ground water exists and is always equal to atmospheric pressure
|
|
|
|
Surface run-off is:
|
water that flows over land before reaching a river, lake, or ocean.
It occurs when precipitation falls faster than the soil can absorb it and/or when the soil becomes saturated with precipitation. |
|
|
|
Human activity, such as creating impermeable surfaces and heavily tilled farmland, can increase surface run-off. How is this detrimental?
|
- reduces ground water supplies
- triggers increased erosion - siltation - flooding - fertile topsoil is carried away at a higher rate |
|
|
|
When the Earth is closest to the Sun, it is called ______, and happens on what date?
|
perihelion; Jan 2nd
|
|
|
|
When the Earth is farthest from the Sun, it is called _______, and happens on what date?
|
aphelion; July 2nd
|
|
|
|
The shape of the Earth's orbit around the Sun deviates from the shape of a circle only slightly, this deviation is called:
|
eccentricity
|
|
|
|
The axis of the Earth is tilted _____ from the perpendicular.
|
23.45 degrees
|
|
|
|
The tilt of the Earth's axis is known as the:
|
the obliquity of the ecliptic
|
|
|
|
The obliquity of the ecliptic is mainly responsible for:
|
the four seasons of the year, by influencing the intensity of solar rays received by the northern and southern hemispheres.
|
|
|
|
The effect of the Earth's tilt on climate is best demonstrated at the:
|
solstices
|
|
|
|
What are the solstices?
|
The two days of the year when the Sun is farthest from the Earth's equatorial plane.
|
|
|
|
When is the summer solstice and what does it mean for the Earth?
|
Happens in June; the Earth's tilt on its axis causes the northern hemisphere to lean toward the Sun, while the Southern hemisphere leans away.
|
|
|
|
When is winter solstice and what does it mean for the Earth?
|
December; the southern hemisphere leans toward the sun.
|
|
|
|
What time of year is tidal bulge especially strong and why?
|
In Spring, during full moon and new moon; because this is when the Earth, Moon, and Sun are in line.
|
|
|
|
The period when tides are particularly weak are called what, when, and why?
|
Neap tides; when the Moon and the Sun are perpendicular to one another; neap tides occur during quarter moons.
|
|
|
|
The moon orbits the Earth every:
|
27 days
|
|
|
|
The moon is in ________ rotation around the Earth; which means what in terms of how it faces the Earth?
|
synchronous; means the same side of the Moon always faces the Earth.
|
|
|
|
As the Moon orbits the Earth, and the Earth orbits the Sun, what does it mean in terms of how the moon faces the sun.
|
The half that faces the sun changes.
|
|
|
|
When do observers on Earth perceive a full moon?
|
when the Sun and Moon are on opposite sides of the Earth.
|
|
|
|
A gibbous moon is what?
|
is between a full moon and a half moon, or between a half moon and a full moon.
|
|
|
|
How are the Sun, Moon, & Earth oriented during a New Moon?
|
The Sun and the Moon are on the same side of Earth.
The illuminated half of the moon is facing away from the Earth. |
|
|
|
The time between each full moon is approximately...
|
29.53 days.
|
|
|
|
Describe a new moon
|
The moon is invisible
|
|
|
|
Describe a waxing crescent
|
The right crescent of the moon is visible
|
|
|
|
Describe a first quarter
|
The right quarter of the moon is visible
|
|
|
|
Describe a waxing gibbous
|
Only the left crescent is not illuminated.
|
|
|
|
Describe a full moon
|
The entire illuminated half of the moon is visible.
|
|
|
|
Describe a waning gibbous
|
Only the right crescent of the moon is not illuminated.
|
|
|
|
Describe a last quarter
|
The left quarter of the moon is illuminated.
|
|
|
|
Describe a waning crescent
|
Only the left crescent of the moon is illuminated.
|
|
|
|
In terms of the sun, by the process of nuclear _____, _____ gas is converted to _____ gas.
|
fusion; hydrogen, helium
|
|
|
|
Parts of the Sun include : (4)
|
1. core
2. photosphere 3. chromosphere 4. corona |
|
|
|
Describe the core of the Sun:q
|
the inner portion of the sun where fusion takes place
|
|
|
|
Describe the photosphere of the Sun:
|
considered the surface of the sun which produces sunspots
|
|
|
|
Describe the chromosphere of the Sun:
|
hydrogen gas causes this portion to be red in color
- also found here are solar flares |
|
|
|
Describe the corona of the Sun:
|
the transparent area of sun visible only during a total eclipse
|
|
|
|
Solar flares do what?
|
Produce excited protons and electrons that shoot outward from the chromosphere at great speeds reaching Earth.
These particles disturb radio reception and also affect the magnetic field on Earth. |
|
|
|
To become a star, a body must have:
|
mass greater than 7 percent of the mass of the Sun.
|
|
|
|
Bodies that have mass less than 7 % of the mass of the Sun, become:
|
planets or brown dwarfs
|
|
|
|
The largest accurately determined stellar mass is of a star called______, and is _____ times that of the Sun.
|
V382 Cygni; 27 times
|
|
|
|
Astronomers measure the brightness of a star by measuring its _______ and _______.
|
magnitude and luminosity
|
|
|
|
Magnitude allows astronomers to:
|
rank how bright, comparatively, different stars appear to humans.
|
|
|
|
Stars with _____ magnitudes are the brightest.
|
negative
|
|
|
|
Magnitude is given in terms of _____ and ______ values.
|
absolute and apparent
|
|
|
|
Luminosity of a star is its ________.
|
intrinsic brightness
|
|
|
|
A star's absolute luminosity, or intrinsic brightness, is the:
|
total amount of energy radiated by the star per second, and is expressed in units of watts.
|
|
|
|
Who was the first person to use telescopes to observe the solar system?
|
Galileo
|
|
|
|
Galileo invented the first:
|
refracting telescope
|
|
|
|
A refracting telescope uses:
|
lenses to bend light rays to focus the image.
|
|
|
|
Sir Isaac Newton invented the:
|
reflecting telescope
|
|
|
|
the reflecting telescope uses:
|
mirrors to gather light rays on a curved mirror which produces a small focused image.
|
|
|
|
The Hubble Space telescope uses:
|
a single-reflector mirror.
|
|
|
|
A radio telescope...
|
collects invisible radio waves created by the sun and stars
|
|
|
|
Radio telescopes consists of:
|
a reflector or dish with special receivers.
- can operate at any time of day and under any weather conditions - ability to detect objects from great distances in space. |
|
|
|
The three formulas astronomers use for calculating distances in space are:
|
1. the astronomical unit (AU)
2. the light year (ly) 3. the parsec (pc) |
|
|
|
The distance between the Earth and the Sun is about:
|
150 x 10^6 km, this is an astronomical unit
|
|
|
|
Astronomical units are used to measure:
|
distances within the solar system
|
|
|
|
The distance light travels in one year is a light year and is equal to:
|
9.5 x 10^12 km
|
|
|
|
a parsec is equal to:
|
3.26 light years
|
|
|
|
There are approximately how many AUs in one light year?
|
63,000 AUs
|
|
|
|
A spectroscope is:
|
a device that is used to separate white light into a series of different colors by wave lengths.
|
|
|
|
A spectrograph is:
|
a device that can photograph a spectrum
|
|
|
|
Wavelengths of light are arranged to form an:
|
electromagnetic spectrum
|
|
|
|
Spectroscopes are useful for measuring what in space?
|
- Spectra
- temperatures - pressures - movement of stars (toward or away from Earth) |
|
|
|
Describe the planet Mercury:
|
- closest planet to the sun
- surface has craters & rocks - atmosphere is composed of hydrogen, helium, and sodium. |
|
|
|
Describe the planet Venus:
|
- has a slow rotation (compared to Earth)
- rotates in opposite direction from other planets - the surface is not visible due to the extensive cloud cover - the atmosphere is composed mostly of carbon dioxide; much of the clouds include sulfuric acid droplets |
|
|
|
Describe the planet Mars:
|
- contains numerous craters
- active & extinct volcanoes - red surface is from iron oxide in its soil - atmosphere is similar to Earth's - mars has polar regions with ice caps composed of water. |
|
|
|
Describe the planet: Jupiter:
|
- largest planet
- has 16 moons - colored bands from descending gases, caused by heat from core - has a strong magnetic field - has a Great Red Spot that is thought to be a hurricane type cloud |
|
|
|
Describe the planet Saturn:
|
- is the second largest planet
- has rings of ice, rock, & dust - atmosphere composed of hydrogen, methane, helium, ammonia. - has over 20 satellites |
|
|
|
Describe the planet Uranus:
|
- is a gaseous planet
- has 10 dark rings & 15 satellites - hyrdogen, helium, & methane |
|
|
|
Describe the planet Neptune:
|
- a gaseous planet
- hydrogen, helium, methane - 3 rings and 2 satellites |
|
|
|
Describe the planet Pluto:
|
- status as a planet is being reconsidered
- methane, ammonia, frozen water - 1 satellite - revolves around the Sun 250 years |
|
|
|
Asteroids are made of what, and are found where?
|
made of rock; generally found in the region between Mars and Jupiter
|
|
|
|
Comets are:
|
masses of frozen gasses, cosmic dust, and small rocky particles.
|
|
|
|
Comets consist of what parts?
|
a nucleus, a coma, and a tail.
|
|
|
|
A comets tails always...
|
points away from the Sun
|
|
|
|
Halley's Comet returns to Earth's skies every:
|
75 to 76 years
|
|
|
|
Meteoroids are:
|
composed of particles of rock and metal of various sizes
|
|
|
|
A meteor is:
|
a meteoroid that is burning through Earth's atmosphere
|
|
|
|
Meteorites are:
|
meteors that strike the Earth's surface.
|
|
|
|
What is the Oort cloud?
|
It's a hypothetical spherical cloud surrounding our solar system. It extends approx. 3 lys from the Sun.
|
|
|
|
The Oort Cloud is believed to be made up of:
|
materials ejected out of the inner solar system because of interaction with Uranus and Neptune, but is gravitationally bound to the Sun.
|
|
|
|
Explain how the Oort cloud came to be:
|
small objects formed near the giant planets would have been ejected from the solar system by gravitational encounters.
Those that didn't escape entirely formed the Oort cloud. Small objects formed farther out had no such interactions and remained as the Kuiper belt objects. |
|
|
|
The Kuiper belt is:
|
the name given to a vast population of small bodies orbiting the sun beyond Neptune.
|
|
|
|
It's believed that objects in the Kuiper belt are remnants of:
|
the earliest phases of the solar system.
|
|
|
|
Prevailing theory states that scattered disk objects began...
|
as Kiuper belt objects which were scattered through gravitational interactions with the giant planets
|
|
|
|
Constellations are...
|
groups or patterns of stars that astronomers use to as a reference point to locate other stars in the sky.
|
|
|
|
First-magnitude stars are:
|
21 of the brightest stars thta can be seen from Earth.
|
|
|
|
In the northern hemisphere, there are ___ commonly observed first-magnitude stars.
|
15
|
|
|
|
Galaxies are:
|
vast collections of stars
|
|
|
|
Galaxies are classified as:
|
irregular, elliptical, and spiral
|
|
|
|
An irregular galaxy has:
|
no real structured appearance
- most are in their early stages of life. |
|
|
|
An elliptical galaxy consists of:
|
smooth ellipses, containing little dust and gas, but composed of millions or trillion stars.
|
|
|
|
Spiral galaxies are:
|
disk-shaped and have extending arms that rotate around its dense center.
|
|
|
|
Earth's galaxy is what kind of galaxy and where is it found?
|
A spiral galaxy found in the Milky way
|
|
|
|
A pulsar is defined as:
|
a variable radio source that emits signals in very short, regular bursts; it is believed to be a rotating neutron star.
|
|
|
|
A quasar is defined as:
|
an object that photographs like a star but has an exremely large redshift and a variable energy output; it is believed to be the active core of a very distant galaxy.
|
|
|
|
Black holes are defined as:
|
an object that has collapsed to such a degree that light cannot escape from its surface; light is trapped by the intense gravitational field.
|
|
|
|
The two main hypotheses of the origin of the solar system are:
|
1. the tidal hypothesis
2. the condensation hypothesis |
|
|
|
The Tidal hypothesis proposes:
|
that the solar system began with a near collision of the sun and a large star. The great gravitational pull of the large star extracted hot gases out of the sun. The mass from the hot gases started to orbit the sun, which began to cool then condensing into the nine planets. (few astronomers support this scenario)
|
|
|
|
The condensation hypothesis proposes:
|
that the solar system began with rotating clouds of dust and gas. Condensation occurred in the center forming the sun, and the smaller parts of the cloud formed the nine planets. (widely accepted by many astronomers)
|
|
|
|
Two main theories to explain the origins of the universe:
|
1. Big Bang
2. Steady-State theory |
|
|
|
The Big Bang theory states:
|
that the universe originated from a magnificent explosion spreading matter and energy into space. The galaxies formed from this material as it cooled during the next half-billion years.
|
|
|
|
The Steady-State theory states
|
that the universe is a continuously being renewed. Galaxies move outward and new galaxies replace older galaxies. Astronomers have not found any evidence to prove this theory.
|
|
|
|
Describe the how the future of the universe is hypothesized:
|
the oscillating universe model: the universe will oscillate or expand and contract.
Galaxies will move away from one another and will in time slow down and stop. Then a gradual moving toward each other will again activate the explosion or a big bang. |
|
|
|
Describe the stages of life for a star:
|
starts with a mass of gas and dust that becomes a nebula, then a main sequence star.
Next, it becomes a red giant, then a nova, and then, in its final stages, a white dwarf, a neutron star or a black hole. |
|
|
|
What's a nebula?
|
A cloud of particles of gas and dust that forms from forces of gravity acting on these particles. Nebulas become stars.
|
|
|
|
How do nebulae become stars?
|
Gravity attracts the particles closer together, and as it grows, its temperature increases, eventually resulting in fusion.
|
|
|
|
Why do stars die?
|
They exhaust their hydrogen, and its core collapses
|
|
|
|
A nova is:
|
an ordinary star that experiences a sudden increase in brightness and then fades back to its original brightness.
|
|
|
|
A supernova is:
|
much brighter than a nova.
|
|
|
|
A neutron star is:
|
the result of mass left behind after a supernova.
|
|
|
|
A blackhole is:
|
a star with condensed matter and gravity so intense thta light cannot escape it.
|
|
|
|
All aspects, whether it be a cell or an ecosystem, have the same requirements to:
|
sustain life
|
|
|
|
_____ make up cells.
|
Organelles
|
|
|
|
_____ make up tissues.
|
cells
|
|
|
|
_____ make up organs.
|
Tissues
|
|
|
|
______ make up organ systems
|
Organs
|
|
|
|
___________ work together to provide life for the organism.
|
Organ systems
|
|
|
|
3 characteristics used to identify living vs. non-living substances:
|
1. Living things are made of cells; they grow, are capable of reproduction.
2. Living things must adapt to environmental changes or perish. 3. Living things carry on metabolic processes. They use and make energy. |
|
|
|
All organic life has a common element:
|
carbon
|
|
|
|
Basic unit of all living things:
|
the cell
|
|
|
|
Two types of cells are:
|
prokaryotic & eukaryotic
|
|
|
|
Prokaryotic cells consist only of:
|
bacteria and blue-green algae.
|
|
|
|
______ were most likely the first cells and date back in the fossil record to ______ years ago.
|
bacteria; 3.5 billion
|
|
|
|
Some important facts that put prokaryotic cells in their own group:
|
1. they have not defined nucleus or nuclear membrane. The DNA and ribosomes float freely within the cell.
2. They have a thick cell wall. 3. The cell walls contain amino sugars (glycoproteins). 4. Penicillin disrupts the cell walls. 5. Some have capsule made of polysaccharides which mack the bacteria sticky. 6. Some have pili, which is a protein strand. This also allows for attachment of the bacteria and may be used for sexual reproduction. 7. Some have flagella for movement. |
|
|
|
Eukaryotic cells are found in: (3)
|
- protists
- fungi - animals |
|
|
|
Some features of eukaryotic cells include:
|
1. they are usually larger than prokaryotic cells.
2. They contain many organelles, which are membrane bound areas for specific cell functions. 3. They contain a cytoskeleton which provides a protein framework for the cell. 4. They contain cytoplasm to support the organelles and contain the ions and molecules necessary for cell function. |
|
|
|
Nucleus is the _____ of the cell.
|
brain
|
|
|
|
The cell nucleus contains:
|
- chromosomes
- chromatin - nucleoli - nuclear membrane |
|
|
|
Describe chromosomes:
|
DNA, RNA and proteins tightly coiled to conserve space while providing a large surface area.
|
|
|
|
Describe chromatin:
|
loose structures of chromosomes. Chromosomes are called chromatin when the cell is not dividing.
|
|
|
|
Describe nucleoli:
|
where ribosomes are made. These are seen as dark spots in the nucleus.
|
|
|
|
Describe nuclear membrane:
|
contains pores which let RNA out of the nucleus. The nuclear membrane is continuous with the endoplasmic reticulum which allows the membrane to expand or shrink if needed.
|
|
|
|
Name the 9 major organelles of a cell:
|
1. Nucleus
2. Ribosomes 3. Endoplasmic Reticulum 4. Golgi Complex or Golgi Apparatus 5. Lysosomes 6. Mitochondria 7. Cell wall 8. Vacuoles 9 Cytoskeleton |
|
|
|
Ribosomes are:
|
the site of protein synthesis.
|
|
|
|
Describe ribosomes:
|
may be free floating in the cytoplasm or attached to the endoplasmic reticulum.
|
|
|
|
There may bee up to a _________ ribosomes in a cell, depending on how much _______ is made by the cell.
|
half-million; protein
|
|
|
|
Form and function of Endoplasmic reticulum:
|
folded and provide a large surface area.
They are the "roadway" of the cell and allow for transport of materials. |
|
|
|
What's the lumen and where is it found?
|
The lumen of the endoplasmic reticulum helps keep materials out of the cytoplasm and headed in the right direction.
|
|
|
|
The endoplasmic reticulum is capable of building:
|
new membrane material
|
|
|
|
There are 2 types of endoplasmic reticulum:
|
1. Smooth
2. Rough |
|
|
|
Describe Smooth Endoplasmic Reticulum:
|
contain no ribosomes on their surface.
|
|
|
|
Describe Rough Endoplasmic Reticulum:
|
contain ribosomes on their surface.
|
|
|
|
Rough Endoplasmic Reticulum is abundant in cells that:
|
make many proteins, like the pancreas, which produces many digestive enzymes.
|
|
|
|
Form and function of Golgi Complex or Golgi apparatus:
|
This structure is stacked to increase surface area.
|
|
|
|
Lysosomes are found mainly in:
|
animal cells
|
|
|
|
Function of lysosomes:
|
contain digestive enzymes that break down: food, substances not needed, viruses, damaged cell components, and eventually itself.
|
|
|
|
What organelle is believed to be responsible for the aging process?
|
lysosomes
|
|
|
|
Form and Function of Mitochondria
|
Large organelles that contain folds
make ATP to supply energy to the cell. |
|
|
|
What cells have many mitochondria and why?
|
Muscle cells; because they use a great deal of energy
|
|
|
|
In terms of mitochondria, cistae are:
|
the folds inside the mitochondria
|
|
|
|
In terms of mitochondria, what is the function of cistae?
|
The provide a large surface where the reactions of cellular respiration occur.
|
|
|
|
Mitochondria have their own:
|
DNA
|
|
|
|
Mitochondria DNA is used for what?
|
makes Mitochondria capable of reproducing themselves if a greater demand is made for additional energy
|
|
|
|
Mitochondria are found in all cells except:
|
bacteria
|
|
|
|
What are 3 special organelles associated with energy in plants:
|
- chloroplasts
- chromoplasts - amyloplasts |
|
|
|
Chloroplasts are:
|
green, function in photosynthesis. They are capable of trapping sunlight.
|
|
|
|
Function of chromoplasts:
|
to make and store yellow and orange pigments; the provide color to leaves, flowers, and fruits.
|
|
|
|
Function of amyloplasts:
|
store starch and are used as a food reserve. They are abundant in roots like potatoes.
|
|
|
|
Cell Wall is only found in ______ cells.
|
plant
|
|
|
|
the cell wall is composed of: (2)
|
cellulose and fibers
|
|
|
|
Function of cell wall:
|
thick enough for support and protection, yet porous enough to allow water and dissolved substances to enter.
Cell walls are cemented to each other. |
|
|
|
Form and function of vacuoles:
|
Vacuoles are very large in plants. This allows them to fill with water in order to provide turgor pressure.
They hold stored food and pigments. |
|
|
|
Lack of what kind of pressure causes plants to wilt?
|
turgor
|
|
|
|
Form and function of the cytoskeleton:
|
composed of protein filaments attached to the plasma membrane and organelles.
They provide a framework for the cell and aid in cell movement. |
|
|
|
Cytoskeletons are constantly:
|
changing shape and moving about.
|
|
|
|
Three types of ____ make up cytoskeletons, name them:
|
fibers:
1. Microtubules 2. Intermediate Filaments 3. Microfilaments |
|
|
|
The largest cytoskeleton fiber is the:
|
microtubule
|
|
|
|
What fiber makes up cilia and flagella?
|
microtubules
|
|
|
|
What are some examples of flagella & cilia?
|
- Sperm use flagella
- tracheal cilia sweep fluids and foreign particles out of the airway. |
|
|
|
Centrioles are composed of what fiber?
|
microtubules
|
|
|
|
Function of centrioles:
|
the form the spindle fibers that pull the cell apart into two cells during cell division.
|
|
|
|
Centrioles are not found in the cells of:
|
higher plants
|
|
|
|
Form and function of Intermediate Filaments
|
they are smaller than microtubules but large than microfilaments.
They help the cell to keep its shape. |
|
|
|
Smallest of the three cytoskeleton fibers:
|
microfilaments
|
|
|
|
Microfilaments are made of:
|
actin and small amounts of mysosin (like in muscle cells)
|
|
|
|
Function of microfilaments in cell movement:
|
- cytoplasmic streaming
- endocystosis, - ameboid movement. |
|
|
|
Function of microfilament in cell division:
|
this structure pinches the two cells apart after cell division, forming two cells.
|
|
|
|
Mitosis is the:
|
division of somatic cells
|
|
|
|
Meiosis is the:
|
division of sex cells
|
|
|
|
Summarize the major processes of Mitosis: (4)
|
- Division of somatic cells
- two cells result from each division - Chromosome number is identical to parent cells. - For cell growth and repair |
|
|
|
Summarize the major proceses of Meiosis: (4)
|
- division of sex cells
- 4 cells or polar bodies result from each division - chromosome number is half the number of parent cells - recombinations provide genetic diversity |
|
|
|
What's a gamete?
|
a sex cell or germ cell; eggs and sperm.
|
|
|
|
What are homologues?
|
chromosomes that contain the same information.
They are the same length and contain the same genes. |
|
|
|
A diploid is?
|
2n number;
diploid chromosomes are a pair of chromosomes (somatic cells). |
|
|
|
A haploid is?
|
1n number;
haploid chromosomes are a half of a pair (sex cells). |
|
|
|
The 2 stages of the cell life cycle are:
|
- interphase
- mitotic division |
|
|
|
What are the three steps of interphase?
|
1. G1 (growth) period
2. S (synthesis) period 3. G2 (growth) period |
|
|
|
What happens in G1?
|
the cell is growing and metabolozing
|
|
|
|
What happens in S period?
|
new DNA and enzymes are being made
|
|
|
|
What happens in G2?
|
where new proteins and organelles are being made to prepare for cell division.
|
|
|
|
The mitotic stage consists of the:
|
stages of mitosis and the division of the cytoplasm.
|
|
|
|
The stages of mitosis, in order, are:
|
IPMAT:
1. Interphase 2. Prophase 3. Metaphase 4. Anaphase 5. Telephase |
|
|
|
Describe what happens during Interphase:
|
(Interphase is technically not a stage of mitosis)
- chromotin is loose - chromosomes are replicated - cell metabolism is occuring |
|
|
|
Describe what happens during Prophase:
|
** unlike interphase, once a cell enters prophase, it proceeds through the following steps without stopping.
- the chromotin condenses to become visible chromosomes - the nucleolus disappears and the nuclear membrane breaks apart - Mitotic spindles form which will eventually pull the chromosomes apart. - the cytoskeleton breaks down and the spindles are pushed to the poles or opposite ends of the cell by the action of centrioles |
|
|
|
Describe what happens during Metaphase:
|
- kinetechore fibers attach to the chromosomes which causes the chromosomes to line up in the center of the cell.
|
|
|
|
Describe what happens during Anaphase:
|
- centromeres split in half and homologous chromosomes separate.
- the chromosomes are pulled to the poles of the cell, with identical sets at either end. |
|
|
|
Describe what happens during Telophase:
|
- two nuclei form with a full set of DNA that is identical to the parent cell.
- the nucleoli become visible and the nuclear membrane reassembles. - in a plant: a cell plate is visible; whereas, in an animal, a cleavage furrow is formed. - the cell is pinched into 2 cells. - cytokinesis, or division, of the cytoplasm and organelles occurs. |
|
|
|
Meiosis contains the same 5 stages of mitosis, but is what and why?
|
It's repeated, in order to reduce the chromosome by one half.
|
|
|
|
Why do you want to halve the number or chromosomes during meiosis?
|
This way, when the sperm and egg join during fertilazation, the haploid number is reached.
- in meiosis I: cells remain diploid - in meiosis II: cells are haploid |
|
|
|
Describe what happens during Prophase I?
|
- replicated chromosomes condense and pair with homologues, forming a tetrad.
- Crossing over (the exchange of genetic material between homologues to further increase diversity) occurs during this phase. |
|
|
|
What is a tetrad?
|
when chromosomes pair with homologues
|
|
|
|
At what phase is genetic material exchanged in cell division?
|
Prophase I
|
|
|
|
Describe what happens during Metaphase I:
|
homologues sets attach to spindle fibers after lining up in the middle of the cell.
|
|
|
|
Describe what happens during Anaphase I:
|
sister chromatids remain joined and move to the poles of the cell.
|
|
|
|
Describe what happens during Telophase I:
|
two new cells are formed, chromosome number is still diploid.
|
|
|
|
Describe what happens during Prophase II:
|
chromosomes condese
|
|
|
|
Describe what happens during Metaphase II:
|
- spindle fibers form again
- sister chromatids line up in the center of the cell, centromeres divide and sister chromatids separate. |
|
|
|
Describe what happens during Anaphase II:
|
separated chromosomes move to opposite ends of the cell.
|
|
|
|
Describe what happens during Telophase II:
|
- 4 haploid cells form for each original sperm germ cell.
- 1 viable egg cell gets all the genetic information & 3 polar bodies from with no DNA - The nuclear membrane reforms and cytokinesis occurs. |
|
|
|
Mutations may be errors in:
|
replication, or a spontaneous rearrangement of one or more segments by factors like: radioactivity, drugs, or chemicals.
|
|
|
|
Inheritable changes in DNA are called:
|
mutations
|
|
|
|
What kind of cells can mutations occur on?
|
Somatic or sex cells
|
|
|
|
What type of cells are mutations most dangerous and why?
|
Sex cells, since they contain the basis of all information for the developing offspring.
|
|
|
|
Mutations are not always bad, they are the basis for what?
|
evolution
|
|
|
|
What are the 6 mutation types?
|
1. duplication
2. Inversion 3. Deletion 4. Insertion or translocation 5. Breakage 6. Nondisjunction |
|
|
|
In terms of mutation, describe duplication:
|
one base is repeated
|
|
|
|
In terms of mutation, describe inversion
|
a segment of the DNA sequence is flipped around
|
|
|
|
In terms of mutation, describe deletion
|
a base is left out
|
|
|
|
In terms of mutation, describe Insertion or translocation:
|
a segment from another place on the DNA is inserted in the wrong place
|
|
|
|
In terms of mutation, describe breakage
|
a piece is lost
|
|
|
|
In terms of mutation, describe nondisjunction
|
this occurs during meiosis when chromosomes fail to separate properly.
One sex cell may get both genes and another may get more. Depending on the chromosome, it may not be serious. Offspring end up with either an extra chromosome or are missing one. |
|
|
|
What mutation type causes Down Syndrome? What's going on with the chromosomes?
|
Nondisjunction;
3 of chromosome #21 are present. |
|
|
|
Recognized as the father of genetics:
|
Gregor Mendel
|
|
|
|
In his work during the _____, ____ realized there were factors that transferred from parent to their offspring?
|
1800s; Gregor Mendel
|
|
|
|
What are the 3 Mendelian Laws of genetics?
|
1. Law of Dominance
2. Law of Segregation 3. Law of Independent assortment |
|
|
|
What is Mendel's law of dominance? Give an example.
|
in a pair of alleles, one trait may cover up the allele of the other trait.
example: brown eyes are dominant to blue eyes |
|
|
|
What is Mendel's law of segregation? Give meiosis example.
|
Only one of the two possible alleles from each parent is passed on to the offspring from each parent.
Example: during meiosis, the haploid number insures that half the sex cells get one allele, half get the other. |
|
|
|
What is Mendel's law of Independent Assortment? Give an analogy
|
alleles sort independently of each other.
Many combinations are possible depending on which sperm ends up with which egg. Compare this to the many combinations of hands possible when dealing a deck of cards. |
|
|
|
A monohybrid cross is:
|
a cross using only one trait.
|
|
|
|
A dihybrid cross is:
|
a cross using two traits.
|
|
|
|
A punnet square is:
|
used to show the possible ways that genes combine and indicate probability of the occurrence of a certain genotype or phenotype.
|
|
|
|
A dominant trait is:
|
the stronger of two traits; dominant genes, if present, will be expressed.
|
|
|
|
A recessive trait is:
|
the weaker of the two traits. It may only be expressed if two recessive genes are present.
|
|
|
|
Incomplete dominance means:
|
neither gene masks the other; a new phenotype is formed.
Heterozygous/homozygous genes |
|
|
|
When is a new phenotype formed?
|
Incomplete dominance, meaning neither gene masks the other.
or, Codominence |
|
|
|
In codominance:
|
genes may form new phenotypes
example: blood types: A, B, O. O is recessive. A & B are both dominant. |
|
|
|
Genotype refers to:
|
all the genes the organism has.
|
|
|
|
Phenotype refers to:
|
how the trait is expressed in the organism
|
|
|
|
Linkage occurs when:
(give an example) |
genes are on the same chromosome and usually appear together unless crossing over has occurred in meiosis.
Blue eyes and blonde hair are linked genes |
|
|
|
Lethal alleles are:
(give some examples) |
- usually recessive due to the early death of the offspring.
- usually the coding for an important protein is affected. - if a 2:1 ratio of alleles is found in offspring, a lethal gene combination is usually the reason. example: sickle cell anemia, Tay-Sachs, cystic fibrosis. |
|
|
|
What is, usually, the reason for a 2:1 ratio of alleles?
|
A lethal gene combination.
|
|
|
|
Inborn errors of metabolism occur:
(give examples) |
when a protein is affect is an enzyme.
examples: PKU & albinism |
|
|
|
Polygenic characters occurs:
(give example) |
when many alleles code for a phenotype. There may be as many as twenty genes that code for skin color.
- This is why there is such a variety of skin tones. - A couple of medium height may have a very tall offsping. |
|
|
|
Sex-influenced traits are:
(give examples) |
traits influenced by the sex hormones.
example: testosterone influences the gene that expresses male pattern baldness. |
|
|
|
What combination makes up a chromosome?
|
DNA & protein
|
|
|
|
The modern definition of a gene: it's a...
|
unit of genetic information
|
|
|
|
DNA controls the synthesis of what? Thereby controlling what?
|
protein; controlling the total cell activity
|
|
|
|
DNA capable of making copies of...
|
itself
|
|
|
|
Review DNA structure: (4 points)
|
1. made of nucleotides
2. consists of a sugar/phosphate backbone which is covalently bonded. 3. the amount of adenine = thymine, and cytosine=guanine 4. The shape is that of twisted ladder, called a double helix. |
|
|
|
Describe the chemical make-up of DNA nucleotides: (3 points)
|
- a five carbon sugar
- phosphate group, - and nitrogen base (either: adenine, guanine, cytosine, or thymine). |
|
|
|
______ control each step of the replication of DNA.
|
Enzymes
|
|
|
|
Describe the DNA replication process:
|
- hyrdogen bonds between the bases break and serve as a pattern for replication.
- Free nucleotides found inside the nucleus join on to form a new strand. - 2 new pieces of DNA are formed which are identical |
|
|
|
What makes the DNA replication process so accurate?
|
Enzymes (polymerases) proofread the molecule.
|
|
|
|
What's the mistake ratio of DNA replication?
|
1 for every billion nucleotides added.
|
|
|
|
In eukaryotes, DNA replication occurs in:
|
many places along the DNA at once, like a broken zipper.
|
|
|
|
In prokaryotic circular plasmids, DNA replication begins...
|
at a point on the plasmid and goes in both directions until it meets itself.
|
|
|
|
What kind of rules are important in determining a new strand of DNA sequence?
|
base paring rules
|
|
|
|
Base pairing rules are important in:
|
determining a new strand of DNA sequence.
|
|
|
|
The DNA nucleotides are:
|
- A (adenine)
- G (guanine) - C (cytosine) - T (thymine) |
|
|
|
The rule of DNA nucleotides is:
|
- A bonds with T
- C bonds with G |
|
|
|
In DNA replication, when the ladder separates, what happens?
|
the new nucleotides (AGCT) attach to the old DNA, and the DNA doubles.
|
|
|
|
The 3 types of RNA are:
|
1. Messenger (mRNA)
2. Transfer (tRNA) 3. Ribosomal (rRNA) |
|
|
|
Why is protein synthesis important?
|
for growth and repair of the organism
|
|
|
|
Protein synthesis is the process that allows:
|
the DNA code to be read and carried out of the nucleus into the cytoplasm in the form of RNA.
|
|
|
|
What is RNA?
|
its the form of DNA as it is carried out of the nucleus into the cytoplasm, for the purpose of having its code read.
|
|
|
|
Where are ribosomes found?
|
In the cytoplasm, at the sites of protein synthesis.
|
|
|
|
Ribsomes are the components of cells that:
|
make protein from all amino acids.
|
|
|
|
The function of messenger RNA:
|
copies the code from DNA in the nucleus and takes it to the ribosomes in the cytoplasm.
|
|
|
|
The function of transfer RNA:
|
free floating in the cytoplasm, it's job is to carry and position amino acids for assembly on the ribosome.
|
|
|
|
The function of ribosomal RNA:
|
found in the ribosomes, they make a place for the proteins to be made.
|
|
|
|
RNA's function is to assist in the:
|
building of proteins
|
|
|
|
The transcription phase allows for:
|
the assembly of mRNA
|
|
|
|
Transcription phase occurs in:
|
the nucleus where the DNA is found.
|
|
|
|
During the transcription phase...
|
the DNA splits open and an enzyme reads the code and "transcribes" the sequence onto a single strand of mRNA.
Example: transcribes DNA nucleotides to RNA, like: T to A; A to U; C to G; and so on. |
|
|
|
What replaces thymine in RNA?
|
uracil
|
|
|
|
In protein synthesis, a codon is:
|
a group of 3 bases
|
|
|
|
The codon will eventually code for a specific _____ ______, to be...
|
a specific amino acid to be carried to the ribosome
|
|
|
|
In protein synthesis, start and stop codons do what?
|
begin the building of the protein; and end transcription.
|
|
|
|
In protein synthesis, when the stop codon is reached, what happens to the mRNA?
|
the mRNA separates from the DNA and leaves the nucleus from the cytoplasm.
|
|
|
|
the translation phase is:
|
the assembly of the amino acids to build the protein.
|
|
|
|
Translation phase occurs where?
|
in the cytoplasm.
|
|
|
|
In the translation phase, what is translated and for what purpose?
|
the nucleotide sequence is translated.
to choose the correct amino acid sequence. |
|
|
|
What translates the code at the ribosome?
|
rRNA
|
|
|
|
What's inherent in tRNA that allows it to do what?
|
it contains an anticodon, to seek out the correct amino acid and bring it back to the ribosome.
|
|
|
|
The whole protein synthesis process is accomplished through the assistance of ________.
|
activating enzynmes
|
|
|
|
Each of the twenty amino acids has it's own _______.
|
enzyme
|
|
|
|
In terms of protein synthesis what does enzyme do?
|
They bind the amino acid to the tRNA.
|
|
|
|
During protein synthesis, when the amino acids get close to each other on the ribosome, they...
|
bond together using peptide bonds.
|
|
|
|
What are nonsense codons?
|
the start and stop codons
|
|
|
|
There is ____ start codon(s), what is/are it/they?
|
1; AUG
|
|
|
|
There are/is ____ stop codon(s). What is/are it/they?
|
3; UAA, UGA, UAG
|
|
|
|
Who is called the father of taxonomy?
|
Carolus Linnaeus
|
|
|
|
What's taxonomy?
|
the science of classification
|
|
|
|
Linnaeus's based his system on...
|
morphology
|
|
|
|
What is phylogeny?
|
taxonomy based on evolutionary relationships.
|
|
|
|
What classification system does modern taxonomy use?
|
binomial nomenclature
|
|
|
|
In binomial nomenclature, every species have a _____ word name. Name each part.
|
2 parts; genus is the first part, species is the second.
|
|
|
|
Name the levels of taxonomy, from top to bottom.
|
- Kingdom
- Phylum - Subphylum - Class - Order - Family - Genus - Species |
|
|
|
Characterize Kingdom Monera. Give examples.
|
Are prokaryotic, unicellular organisms, and have no nucleus.
Examples: bacteria & blue green algae. |
|
|
|
Bacteria are classified according to their _________.
|
Morphology (shape)
|
|
|
|
Morphology of rod shaped bacteria:
|
bacilli
|
|
|
|
Morphology of round shaped bacteria:
|
cocci
|
|
|
|
Morphology of spiral shaped bacteria:
|
spirilla
|
|
|
|
The Gram stain is:
|
a staining procedure used to identify bacteria.
|
|
|
|
Describe the kinds of results you can get from a Gram stain:
|
Gram-positive: bacteria pick up the stain and turn purple.
Gram-negative: bacteria do not pick up the stain and are pink in color. |
|
|
|
Characterize Kingdom Protista, and give examples.
|
Are eukaryotic, unicellular, some are photosynthetic, some are consumers.
|
|
|
|
How do microbiologists classify Protista?
|
By their methods of:
- locomotion, - reproduction, - how the organism obtains its food. |
|
|
|
Protista reproduce by _______, which is:
|
binary fission; dividing in half and is asexual.
|
|
|
|
In the Protista kingdom, all new organisms are:
|
are exact clones of the parent.
|
|
|
|
In the Protista Kingdom, bacteria can reproduce sexually, through _______, which is:
|
conjugation; where genetic material is exchanged.
|
|
|
|
Saprophytes are:
|
consumers that live off dead or decaying material.
|
|
|
|
Characterize the Kingdom Fungi:
|
includes eukaryotic, multicellular, and absorptive consumers, which contain a chitin cell wall.
|
|
|
|
What are the 4 groups of Kingdom Plantae:
|
- Nonvascular
- Vascular - Gymnosperms - Angiosperms |
|
|
|
Characterize nonvascular plants:
|
- are small in size
- do not require vascular tissue (xylem & phloem) - have no true leaves, stems, or roots - a division of this group possess rhizoids |
|
|
|
Why don't nonvascular plants need vascular tissue?
|
because individual cells are close to their environment.
|
|
|
|
What division of nonvascular plants has rhizoids?
|
Division Bryophyta
|
|
|
|
Characterize Division Bryophyta of nonvascular plants. Give examples.
|
- have a dominant gametophyte generation
- possess rhizoids - moisture in their environment is required for reproduction and absorption examples: mosses and liverworts |
|
|
|
Characterize Vascular plants:
|
- have vascular tissue
- all vascular plants have a dominant sporophyte. |
|
|
|
What are the 3 divisions of vascular plants:
|
1. Lycophyta
2. Sphenophyta 3. Pterophyta |
|
|
|
What the function of vascular tissue in vascular plants? What are the two types? What does it allow the plant to do?
|
Vascular tissues: Xylem & Phloem allow plants to grow in size, by transporting water and minerals to the top of the plant
|
|
|
|
Characterize Division Lycophyta. Give example.
|
- reproduce with spores and require water from reproduction.
example: club mosses |
|
|
|
Characterize Division Sphenophyta. Give example
|
- have small, needle-like leaves and rhizoids and require moisture for reproduction.
example: horsetails |
|
|
|
Characterize Pterophyta. Give example.
|
- reproduce with spores and flagellated sperm.
- these plants have a true stem and need moisture for reproduction. example: ferns |
|
|
|
What are gymnosperms?
|
- the first plants to evolve with seeds, which made them less dependent on water to assist in reproduction.
- have cones to product seeds |
|
|
|
What are the 4 divisions of gymnosperms?
|
- Cycadophyta
- Ghetophyta - Coniferophyta - Ginkgophyta |
|
|
|
Characterize Cycadophyta. Give example.
|
Look like palms with cones.
example: cycads |
|
|
|
Characterize Ghetophyta.
|
plants living in the desert
|
|
|
|
Characterize Coniferophytoa. Give example.
|
Have needles and cones.
Example: pines |
|
|
|
Characterize Ginkgophyta.
|
have ginkgo as its only member
|
|
|
|
Characterize Angiosperms:
|
- are the largest group in the plant kingdom.
- they are flowering plants and produce true seeds from reproduction. |
|
|
|
What division are angiosperms?
|
Anthophyta
|
|
|
|
What are the 5 groups of the Kingdom Animalia?
|
- Annelida
- Mollusca - Anthropoda - Echinodermata - Chordata |
|
|
|
Annelida are:
|
segmented worms
|
|
|
|
Annelids have specialized _____.
|
tissue
|
|
|
|
The circulatory system of Annelids is: (2 points)
|
closed with blood vessels
|
|
|
|
The excretory organs of Annelids are called:
|
nephrida
|
|
|
|
Sexually, Annelids are _______, which means:
|
hermaphrodidic; each worm fertilizes the other upon mating.
|
|
|
|
Annelida have a ______ skeleton.
|
hydrostatic
|
|
|
|
Annelida have ______ and _____ muscles.
|
circular & longitudinal
|
|
|
|
Examples of Mollusca:
|
clams, octopus, soft bodied animals.
|
|
|
|
Characterize Mollusca.
|
- have a muscular foot for movement.
- open circulatory system - breath through gills - most can make shells - have sinuses used for bathing the body regions. |
|
|
|
Examples of Arthropoda:
|
- Insects
- Crustaceans - Spiders |
|
|
|
Anthropoda is the ______ group of the animal kingdom. What percent?
|
largest, 85%
|
|
|
|
Animals of arthropoda possess ______ made of _____.
|
exoskeleton, chitin
|
|
|
|
What are the 4 stages of insect development?
|
1. egg
2. larva 3. pupa 4. adult |
|
|
|
Ways Anthropods breath: (3)
|
- gills
- tracheae - book lungs |
|
|
|
Ways Anthropods move: (3)
|
- swim
- fly - crawl |
|
|
|
Successful as a phylum, habitats of arthropods can be said to be:
|
diverse
|
|
|
|
Examples of Echinodermata:
|
- urchins
- starfish |
|
|
|
Characterize echinodermata:
|
- have spiny skin
- marine habitat - have tube feet for locomotion and feeding |
|
|
|
All animals in Chordata have a:
|
notocord or a backbone
|
|
|
|
What are 7 classes of Chordata. Give example of each.
|
1. Agnatha (jawless fish)
2. Chondrichthyes (cartilage fish) 3. Osteichthyes (bony fish) 4. Amphibia (frogs/toads; gills are replaced by lungs during development) 5. Reptilla (snakes; first to lay eggs with a protective covering) 6. Aves (birds; warm-blooded) 7. Mammalia (warm blooded; body hair; bear live young; mammary glands) |
|
|
|
The first life forms on Earth developed about ____ years ago, and were ______.
|
3 billion; protists
|
|
|
|
A protist:
|
a single celled eukaryotic organism.
|
|
|
|
Characterize Protozoans:
|
- protists appearing animal like.
- do not have chloroplasts - classified by the way they move |
|
|
|
3 kinds of animal-like Protozoans:
|
- Amoebas
- paramecium - euglena |
|
|
|
The simplest microorganisms are:
|
bacteria
|
|
|
|
Characterize a bacterial cell.
|
- has a cell wall
- has no nucleus - most do not contain chlorophyll - classified by shape |
|
|
|
Viruses replicate once they:
|
get inside a host cell
|
|
|
|
Characterize viruses
|
- they are not cells
- cannot perform the processes of life - not classified as living organisms - has very few genes (e.g. has 9, compared to 1000s) |
|
|
|
Characterize fungi
|
- different from plants & animals
- can be uni- or multicellular Yeasts are unicellular fungi |
|
|
|
The ____ of the cell is often related to the cell's _______.
|
structure; function
|
|
|
|
Animal cells are ______ cells, that contain a standard set of _______.
|
eukaryotic; organelles
|
|
|
|
How do animal cells differ from plant cells?
|
Animal cells have centrioles.
|
|
|
|
How do plant cells differ from animal cells?
|
Plant cells have cell walls and plastids (including chloroplasts).
|
|
|
|
Name 16 plant tissues:
|
- Xylem
- Phloem - Cortex - Epidermis - Endodermis - Pericycle - Pith - Sclerenchyma cell - Stomata - Guard cells - Palisade mesophyll - Spongy mesophyll - Seed coat - Cotyledon - Endosperm - Apical meristem |
|
|
|
Name 11 reproductive structures of a flower:
|
- Pedical
- Receptacle - Sepals - Petals - Anther - Filament - Stigma - Style - Ovary - Carpel - Stigma |
|
|
|
Function of the Xylem
|
tube-shaped part of plants that transports water and mineral from the roots to the rest of the plant.
|
|
|
|
Function of the Phloem
|
elongated tubes that transports food (glucose) and other nutrients to the rest of the plant
|
|
|
|
Function of the plant Cortex
|
region of the root used for storage of food and water
|
|
|
|
Function of the plant epidermis
|
protective covering of plant
|
|
|
|
Function of the plant endodermis
|
controls movement between the cortex and the cell interior
|
|
|
|
Function of the plant pericycle
|
meristematic tissue which can divide when necessary
|
|
|
|
Function of the plant pith
|
tissue in stems used for storage
|
|
|
|
Function of the sclerenchyma cell
|
a rigid supportive plant cell for stems.
|
|
|
|
Function of the plant stomata
|
Openings on the underside of leaves that let carbon dioxide in and water out (transpiration)
|
|
|
|
Function of the plant guard cells
|
control the size of the stomata. If the plant has to conserve water, the stomata will close.
|
|
|
|
Function of the palisade mesophyll:
|
site of photosynthesis that contains the chloroplasts in leaves
|
|
|
|
Function of the spongy mesophyll
|
plant tissue with open spaces in the leaf that allows for gas circulation.
|
|
|
|
Function of the seed coat
|
protective covering on a seed
|
|
|
|
Function of the cotyledon
|
small seed leaf that emerges when the seed germinates
|
|
|
|
Function of the plant endosperm
|
a nutrient-rich tissue that supplies food in the seed.
|
|
|
|
Function of the apical meristem
|
embryonic plant tissue in an area of cell division allowing for growth
|
|
|
|
Function of the flower's pedical
|
supports the weight of the flower
|
|
|
|
Function of the flower's receptacle:
|
holds the floral organs at the base of the flower
|
|
|
|
Function of the flower's sepals
|
green leaf-like parts that cover the flower prior to blooming
|
|
|
|
Function of the flower's petals
|
- contain coloration by pigments
- purpose is to attract insects to assist in pollination |
|
|
|
Function of the flower's anther
|
male part that produces pollen
|
|
|
|
Function of the flower's filament
|
supports the anther
|
|
|
|
Function of the flower's stamen
|
the filament & anther
|
|
|
|
Function of the flower's stigma
|
female parts that holds pollen grains that come from the male part.
|
|
|
|
Function of the flower's style
|
tube that leads to the ovary
|
|
|
|
Function of the flower's ovary
|
contains the ovules;
|
|
|
|
Function of the flower's carpel
|
the stigma, style, & ovary
|
|
|
|
Photosynthesis is:
|
the process by which plants make carbohydrates from the energy of the sun, carbon dioxide, and water.
|
|
|
|
What molecule does photosynthesis make?
|
carbohydrates
|
|
|
|
What is a waste product of photosynthesis?
|
oxygen
|
|
|
|
Where does photosynthesis occur?
|
in the chloroplast
|
|
|
|
What are the two major steps of photosynthesis?
|
1. (occurs in the light): light is trapped, water is split, and oxygen is given off.
2. The Calvin Cycle: dark reactions: carbon dioxide enters. Energy transferred from NADPH2 and ATP allow for the fixation of carbon into glucose. |
|
|
|
What two molecules allow for the fixation of carbon into glucose, and in what cycle does this happen?
|
NADPH2 and ATP, during the calvin cycle
|
|
|
|
During the light reaction cycle in photosynthesis, what molecules are made, what is reduced to what and by what?
|
- ATP is made
- NADP is reduced to NADPH2 by hydrogen atoms |
|
|
|
Plant cellular respiration happens when:
|
plants break down the products of photosynthesis.
|
|
|
|
Describe what happens, molecularly, during cellular respiration
|
Glucose, with the help of oxygen, breaks down and produces carbon dioxide and water as waste.
|
|
|
|
What percentage of the products of photosynthesis is used by the plant for energy?
|
50 %
|
|
|
|
Transpiration is:
|
the process through which water travels up the xylem of the plant
|
|
|
|
Describe the process of transpiration:
|
- water sticks to itself (cohesion) and to the walls of the xylem (adhesion).
- as water evaporates through the stomata of the leaves, the water is pulled up the column from the roots. |
|
|
|
What environmental factors affect the rate of transpiration?
|
- Heat & wind can increase the rate.
- high humidity will decrease the rate of transpiration. |
|
|
|
What phylum are the flowing plants? What do they produce?
|
Angiosperms. They produce true seeds.
|
|
|
|
Angiosperms reproduce through a method of:
|
double fertilization
|
|
|
|
Describe double fertilization
|
- an ovum is fertilized by two sperm.
- one sperm produces the new plant, the other forms the food supply for the developing plant. |
|
|
|
In terms of angiosperms, the success of plant reproduction involves the seed moving away from the parent plant. Why?
|
- to decrease competition for:
- space - water - minerals |
|
|
|
Animal respiration is simply:
|
taking in oxygen, giving off waste gases
|
|
|
|
Respiration without oxygen is called:
|
anaerobic respiration
|
|
|
|
Another term for anaerobic respiration is:
|
lactice acid fermentation
|
|
|
|
The end products of lactic acid fermentation are:
|
lactic acid & carbon dioxide
|
|
|
|
Animal reproduction can be ______ or ______.
|
asexual or sexual
|
|
|
|
Animal digestion breaks down: (3)
|
- carbohydrates
- fats - proteins |
|
|
|
What catalyst facilitates animal digestion? How?
|
Enzymes help speed up chemical reactions by lowering effective activation energy.
|
|
|
|
In animal digestion, enzyme rate is affected by: (3)
|
- temperature
- pH - amount of substrate |
|
|
|
Saliva contains the enzyme ______ that changes ______ into _______.
|
amylase; starches into sugars
|
|
|
|
Animal circulation refers to the...
|
flow of blood
|
|
|
|
What's the difference between warm and cold blooded animals.
|
Warm blooded animals, their blood temperature remains constant regardless of outside temperature. Cold blooded animals' blood varies with temperature.
|
|
|
|
The function of a skeletal system is:
|
support
|
|
|
|
Animal body size and shape is limited due to:
|
the forces of gravity
|
|
|
|
Three types of muscle tissue:
|
- skeletal
- smooth - cardiac |
|
|
|
Describe skeletal muscle:
|
- movement is voluntary
- attached to bones |
|
|
|
Describe smooth muscle
|
- movement is involuntary
- is found in organs - enables functions e.g. digestion & respiration |
|
|
|
Describe cardiac muscle
|
- it's a specialized type of smooth muscle
|
|
|
|
What is the basic unit of the nervous system?
|
the neuron
|
|
|
|
What do neurons consist of? (5)
|
- axon
- dendrite - the cell body - synapses - myelin sheath |
|
|
|
Function of an axon:
|
- carries impulses away from the cell body
|
|
|
|
Function of the dendrite
|
carries impulses toward the cell body
|
|
|
|
Function of the neuron's cell body:
|
contains the nucleus
|
|
|
|
Function of the synapse:
|
- are spaces between neurons
- chemicals called neurotransmitters are found close to the synapse |
|
|
|
Function of the myelin sheath:
|
- covers the neurons and provides insulation
- composed of Schwann cells |
|
|
|
The function of the digestive system is:
|
to break down food and absorb it into the blood stream where it can be delivered to all cells of the body for use in cellular respiration.
|
|
|
|
the digestive system evolved to do what for the organism:
|
allowed it to be independent of a host
|
|
|
|
Function of the respiratory system
|
- gas exchange of oxygen (needed) and carbon dioxide (waste)
|
|
|
|
Function of the circulatory system
|
- to carry oxygenated blood and nutrients to all cells of the body
- to return carbon dioxide waste to be expelled from the lungs. |
|
|
|
How has the animal circulatory system evolved?
|
from an open system to a closed system with vessels leading to and from the heart.
|
|
|
|
The axial skeleton consists of:
|
the bones of the skull and vertebrate
|
|
|
|
The appendicular skeleton consists of:
|
- bones of the legs, arms, tail, and shoulder girdle.
|
|
|
|
What kind of tissue is bone?
|
connective
|
|
|
|
Parts of the bone: (5)
|
- compact bone
- spongy bone - red marrow - yellow marrow - periosteum |
|
|
|
Function of compact bone:
|
gives bone strength
|
|
|
|
function of spongy bone
|
contains red marrow
|
|
|
|
function of red marrow
|
makes blood cells
|
|
|
|
function of yellow marrow
|
- found in the center of long bones
- stores fat cells |
|
|
|
function of periosteum
|
the protective covering on the outside of bone
|
|
|
|
function of a skeletal joint
|
- defined as a place where two bones meet
- joints enable movement |
|
|
|
function of ligaments
|
- attach bone to bone
|
|
|
|
function of tendons
|
attach bones to muscle
|
|
|
|
Muscle fibers are made of groups of:
|
myofibrils
|
|
|
|
Myofibrils are made up of:
|
sarcomeres
|
|
|
|
Proteins that make up the sarcomere.
|
Actin and myosin
|
|
|
|
Describe the physiology of muscle contraction: (5 points)
|
- a nerve impulse strikes a muscle fiber.
- causing calcium ions to flood the sarcomere. - Calcium ions allow ATP to expend energy. - The myosin fibers creep along the actin, causing the muscle to contract. - Once the nerve impulse has passed, calcium is pumped out and the contraction ends. - |
|
|
|
What does nerve action depend on?
|
it depends on depolarization and an imbalance of electrical charges across the neuron.
|
|
|
|
Explain nerve action...
|
- a polarized nerve has a positive charge outside the neuron
- a depolarized nerve has a negative charge outside the neuron. - Neurotransmitters turn off the sodium pump which results in depolarization of the membrane. - This wave of depolarization (as it moves from neuron to neuron) carries an electrical impulse. - This is actually a wave of opening and closing gates that allows for the flow of ions across the synapse. - Nerves have an action potential. - There is a threshold of the level of chemicals that must be met or exceeded in order for muscles to respond. This is called the all or none response. |
|
|
|
What is the all or none response?
|
Nerves have an action potential. There is a threshold of the level of chemicals that must be met or exceeded in order for muscles to respond.
|
|
|
|
In what state is a neuron that has a positive charge outside the neuron?
|
polarized
|
|
|
|
In what state is a nerve that has a negative charge outside the neuron?
|
depolarized
|
|
|
|
What do neurotransmitters do in a neuron?
|
they turn off the sodium pump which results in depolarization of the membrane
|
|
|
|
What results in the depolarization of the neuron membrane?
|
neurotransmitters turning off the sodium pump.
|
|
|
|
What is the simplest nerve response?
|
the reflex arc
|
|
|
|
Describe what happens during a reflex arc.
|
When a stimulus occurs (touching a hot stove), sensors in the hand sends the message directly to the spinal cord. Bypassing the brain.
- this stimulates motor neurons that contract the muscles of the hand. |
|
|
|
Voluntary nerve responses involve the _______.
|
brain
|
|
|
|
Describe the voluntary nerve response process: (3 steps)
|
- Receptor cells send the message to sensory neurons that lead to association neurons.
- The message is taken to the brain. - Motor neurons are stimulated and the message is transmitted to effector cells that cause the end effect. |
|
|
|
Concerning the organization of the nervous system, the somatic nervous system is controlled ______.
|
conciously
|
|
|
|
The nervous system consists of what two systems?
|
- The central nervous system
- the peripheral nervous system |
|
|
|
Main 2 components of the central nervous system?
|
brain & spinal cord
|
|
|
|
The peripheral nervous system consists of what?
|
nerves that extend from the spinal cord to the muscles.
|
|
|
|
The autonomic nervous system is ________ controlled by the _______ of the brain?
|
unconciously; hypothalamus
|
|
|
|
What are three main organs are controlled by the automatic nervous system?
|
- smooth muscles
- the heart - digestion - other processes |
|
|
|
What 2 nervous systems work in parallel?
|
the sympathetic and parasympathetic.
|
|
|
|
Describe the interaction between the sympathetic and parasympathetic nervous systems.
|
If the sympathetic nervous system stimulates an action, the parasympathetic nervous system would end that action.
|
|
|
|
What process releases neurotransmitters?
|
exocytosis
|
|
|
|
What is the function of neurotransmitters on action?
|
some stimulate action, while others inhibit it.
|
|
|
|
What is the most common neurotransmitter?
|
Acetylcholine
|
|
|
|
Function of acetylcholine
|
it controls muscle contraction and heartbeat.
|
|
|
|
What neurotransmitter is responsible for the "fight or flight" reaction?
|
epinephrine
|
|
|
|
Epinephrine (aka ______) does what to the body?
|
adrenaline; it causes an increase in heart rate and blood flow to prepare the body for action.
|
|
|
|
What are endorphins and enkephalins?
|
Are natural pain killers released during serious injury and childbirth.
|
|
|
|
Lips, cheeks, and tongue do what to food for digestion?
|
form it into a a bolus (ball) to be swallowed
|
|
|
|
From the mouth, food is carried down the _______ by the process of ________.
|
phalanx; peristalsis (wave like contractions)
|
|
|
|
Food enters the stomach from the phalanx through the what gate?
|
the cardiac sphincter.
|
|
|
|
When food reaches the stomach what happens?
|
pepsinogen and hydrochloric acid form pepsin, the enzyme that breaks down proteins.
|
|
|
|
After food is broken down in the stomach, it is turned into what?
|
chyme
|
|
|
|
Small intestine accomplishes large surface area by its length and what structures?
|
villi and microvilli
|
|
|
|
What are the accessory organs?
|
they are not part of the digestive tract, but are necessary in the production of enzymes and bile.
- pancreas - liver |
|
|
|
What makes bile and what is its function?
|
The liver; breaks down and emulsifies fatty acids.
|
|
|
|
In terms of teh respiratory system, what's the function of the nose and mouth?
|
- warms the air
- filters dust and particles |
|
|
|
Describe the structures air passes through after the nose and mouth to the lungs. (3 points)
|
- the travels through the trachea
- trachea splits into 2 bronchial tubes - bronchial tubes divide into smaller and smaller bronchioles in the lungs. |
|
|
|
What is alveoli?
|
- are thin walled sacs that make up the internal surface of the lungs
- they allow for a large surface area for gas exchange. |
|
|
|
The aveoli are lined with what?
|
capillaries
|
|
|
|
What holds the lungs?
|
the thoracic cavity
|
|
|
|
Describe the process of the diaphragm and breathing
|
As the volume of the thoracic cavity decreases, the diaphragm muscle flattens out and inhalation occurs.
- when the diaphragm relaxes, the exhalation occurs. |
|
|
|
Describe the path of blood from unoxygenated to oxygenated to thre rest of the body (7 points)
|
- blood enter through the inferior and superior vena cava.
- enters the right atrium - goes through the tricuspid valve to the right vetricle - to the pulmonary arties - to the lungs where it's oxygenated - returns to the heart through the pulmonary vein into the left atrium - travels through the bicuspid valve to the left ventricle - pumped to all parts of the body through the aorta. |
|
|
|
What is the sinoatrial node? Where is it located? What's it responsible for?
|
- It's the pacemaker of the heart.
- located on the right atrium, it's responsible for contraction of the right and left atrium. |
|
|
|
Function & location of the atrioventricular node (AV node)
|
- located on the left ventricle
- responsible for contraction of the ventricles |
|
|
|
Name 5 types of blood vessels:
|
- arteries
- arterioles - capillaries - venules - veins |
|
|
|
Name 4 components of blood:
|
- Plasma
- erythrocytes - leukocytes - platelets |
|
|
|
Function of and 2 points about arteries
|
- leads away from the heart
- all arteries carry oxygenated blood except the pulmonary artery going to lungs. - arteries are under high pressure |
|
|
|
What are arterioles?
|
arteries branch off to form these smaller passages
|
|
|
|
Describe capillaries:
|
- arterioles branch off to form tiny capillaries that reach every every cell.
- distributes nutrients to cells |
|
|
|
In what blood vessel does blood flow slowest and why?
|
capillaries, because they're the smallest of the vessels.
|
|
|
|
Describe venules: (2 points)
|
- capillaries combine to form larger venules
- these vessels carry waste products from the cells |
|
|
|
describe veins: (3 points)
|
- venules combine to form larger veins, leading back to the heart
- Veins and venules have thinner walls than arteries because they are not under as much pressure. - veins contain valves to prevent the backward flow of blood due to gravity. |
|
|
|
describe plasma: (3 points)
|
- 60% of the blood
- contains salts called electrolytes, and waste. - it is the liquid part of blood |
|
|
|
describe erythrocytes: (2 points)
|
- also called red blood cells
- they contain hemoglobin which carries oxygen molecules |
|
|
|
describe leukocytes: (4 points)
|
- aka white blood cells
- white blood cells are larger than red cells. - they are phagocytic and can engulf invaders - white blood cells are not confined to the blood vessels and can enter the interstitial fluid between cells. |
|
|
|
describe platelets: (2 points)
|
- assist in blood clotting
- are made in the bone marrow |
|
|
|
describe the process of blood clotting: (3 points)
|
- the neurotransmitter that initiates blood vessel constriction following an injury is called serotonin.
- A material called prothrombin is converted to thrombin with the help of thromboplastin. - the thrombin is then used to convert fibrinogen to fibrin, which traps red blood cells to form a scab and stop blood flow. |
|
|
|
What is serotonin? What does it do following an injury?
|
it's a neurotransmitter that initiates blood vessel constriction
|
|
|
|
the immune system protects against disease by:
|
identifying and killing pathogens
|
|
|
|
Intercostal Muscles
(Mammals - Respiratory System) |
- assist in respiration
- intercostal muscles contract = raises rib cage; increased volume of thoracic cavity = expansion of lungs (air drawn in) |
|
|
|
Nonspecific defense mechanisms are considered what kind of a response?
|
a whole body response
|
|
|
|
Nonspecific defense mechanisms are seen as...
|
symptoms of an infection
|
|
|
|
Nonspecific defense mechanisms include: (4)
|
- skin
- mucous membranes - the cells of the blood (white blood cells) - and lymph (macrophages) |
|
|
|
Fever is a result of...
|
- an increase in white blood cells.
- pyrogens are released by white blood cells, which set the body's temperature to a higher temperature. |
|
|
|
What are pyrogens?
|
- Released by white blood cells, they set the body's thermostat
|
|
|
|
What is the purpose of rising body temperature during a fever? (2 points)
|
- inhibits the growth of microorganisms.
- increases metabolism to increase phagocytosis and body repair. |
|
|
|
Function of Specific defense mechanisms?
|
- recognize foreign material and respond by destroying the invader.
|
|
|
|
Specific defense mechanisms are able recognize what? And differentiate between what and what?
|
individual pathogens; foreign material and body cells
|
|
|
|
Function of memory inherent in specific defense mechanism?
|
- memory of invaders provides immunity upon further exposure
|
|
|
|
What are antigens?
|
- any foreign particle that invades the body.
|
|
|
|
Antibodies are (3 points)
|
- manufactured by the body
- recognize & latches onto antigens - hopefully destroys them |
|
|
|
Immunity is...
|
- the body's ability to recognize and destroy an antigen before it causes harm.
|
|
|
|
Differentiate between active and passive immunity.
|
- active immunity develops after recovery from an infectious disease, or vaccination.
- passive immunity may be passed from one individual to another; it is not permanent |
|
|
|
Function of the excretory system:
|
- to rid the body of nitrogenous wastes in the form of urea
- |
|
|
|
What is Antidiuretic hormone (ADH)? What is it's function?
|
- it is made in the hypothalamus and stored in the pituitary.
- it is released when differences in osmotic balance occur, causing more water to be reabsorbed. |
|
|
|
Function of nephrons?
|
- functional unit of excretion that make up the kidneys.
|
|
|
|
The function fot he endocrine system is....
|
to manufacture proteins called hormones.
|
|
|
|
Describe hormone effect on tissues.
|
- hormones are released into the bloodstream and are carried to a target tissue where they stimulate an action.
- hormones may build up over time to cause their effect, as in puberty or the menstrual cycle. |
|
|
|
How are hormones specific to certain tissues?
|
- they fit receptors on the target tissue cell surface
|
|
|
|
Describe how hormones activate genes. (3 points)
|
- when a hormone fits a receptor on a tissue, an enzyme is activated and converts ATP to cyclic AMP.
- Cyclic AMP (cAMP) is a second messenger from the cell membrane to the nucleus. - the genes found in the nucleus turn on or off to cause a specific response. |
|
|
|
What are the 2 classes of hormones?
|
- steroid
- peptide |
|
|
|
Where do steroid hormones come from? What do they do? Which hormones do they include?
|
- come from cholestrol
- cause sexual characteristics and mating behavior. - estrogen & progesterone in females - testosterone in males |
|
|
|
Where are peptide hormones made?
|
In the pituatary, adrenal glands (kidneys)
|
|
|
|
Name 12 peptide hormones
|
- Follicle stimulating hormone (FSH)
- Luteinizing hormone (LH) - Luteotropic hormone (LTH) - Growth hormone (GH) - Antidiuretic hormone (ADH) - Oxytocin - Melatonin - Epinephrine - Thyroxin - Calcitonin - Insulin - Glucagon |
|
|
|
Function of Follicle stimulating hormone:
|
production of sperm and egg cells
|
|
|
|
Function of Luteinizing hormone (LH)
|
functions in ovulation
|
|
|
|
Function of luteotrophic hormone (LTH)
|
assists in production of progesterone
|
|
|
|
Function of Growth hormone (GH)
|
stimulates growth
|
|
|
|
Function of Antidiuretic hormone (ADH)
|
assists in retention of water
|
|
|
|
Function of oxytocin
|
stimulates labor contractions at birth and let down milk
|
|
|
|
Function of melatonin
|
regulates circadian rhythms and seasonal changes
|
|
|
|
Function of Epinephrine:
|
(adrenaline): causes flight or fight reaction of the nervous system
|
|
|
|
Function of Thyroxin
|
increases metabolic rate
|
|
|
|
Function of calcitonin
|
removes calcium from the blood
|
|
|
|
Function of insulin
|
decreases glucose level in blood
|
|
|
|
Function of glucagon
|
increases glucose level in blood.
|
|
|
|
What does it mean that hormones work on a feedback system?
|
The increase or decrease in one hormone may cause the increase or decrease in another.
The release of hormones causes a specific response |
|
|
|
What term means the production of sperm and eggs?
|
gametogenesis
|
|
|
|
what term means the production of sperm cells?
|
spermatogenesis
|
|
|
|
what term means the production of egg cells?
|
oogenesis
|
|
|
|
One spermatogenesis produces...
|
4 sperm
|
|
|
|
In what vessel and in what organ due sperm mature?
|
in seminiferous tubules, located in the testes
|
|
|
|
When is the production of egg cells usually complete?
|
At birth of a female.
|
|
|
|
Meiosis forms _____ ovum with all the cytoplasm and _(#)_ ____ _____ (2 words) which are reabsorbed by the body.
|
1 ovum; 3 polar bodies
|
|
|
|
Mature sperm are found where in relation to the testes?
|
in the epididymous, located on top of the testes
|
|
|
|
After ejaculation, describe the path of the sperm in the male:
|
1. sperm travels up the vas deferens
2. in the vas deferens, they mix with semen made in the prostate and seminal vesicules 3. travel out the urethra |
|
|
|
What stimulates menstruation?
|
progesterone and estrogen hormones
|
|
|
|
Where does egg fertilization normally take place?
|
in the fallopian tubes
|
|
|
|
A fertilized egg is called a:
|
zygote
|
|
|
|
A few days after fertilization, what happens to the zygote?
|
implants in the uterus
|
|
|
|
What does zygote implantation promote?
|
the secretion of human chrorionic gonadotropin (HCG)
|
|
|
|
What does Human chorionic gonadotropin do?
|
Keeps the level of progesterone elevated to maintain the uterine lining in order to feed the developing embryo until the umbilical cord forms.
|
|
|
|
What chemical is detected in pregnancy tests?
|
human chorionic gonadotropin
|
|
|
|
What hormone causes labor contractions and dilation?
|
oxytocin
|
|
|
|
Name 4 important organismal behaviors:
|
- competitive
- instinctive - territorial - mating |
|
|
|
Who discovered cell walls and when?
|
Robert Hooke; 1665
|
|
|
|
In what did Robert Hooke discover cell walls?
|
discovered them in cork
|
|
|
|
What does the cell theory state?
|
that all living things are composed of one or more cells.
|
|
|
|
Which three people contributed to the completion of cell theory?
|
- Robert Hooke
- Antonie van Leeuwenhoek - Rudolf Virchow |
|
|
|
What did Rudolf Virchow do and when?
|
In 1858, he concluded that all cells come from pre-existing cells.
|
|
|
|
What addition has modern cell theory add? (2 points)
|
- energy flow (metabolism and biochemistry) occurs within cells.
- cells contain hereditary information (DNA) that is passed from cell to cell during cell division. |
|
|
|
What are some exceptions to cell theory?
|
- the first cell didn't originate from a pre-existing cell.
- viruses reproduce in a host as if they were living organisms, but are not cells. - there are certain organelles that reproduce independently of the cell (mitochondria & chloroplasts) |
|
|
|
Name 7 sciences that support evolution? Briefly explain how each supports it.
|
- the fossil record
- comparative anatomy - embryology - biogeography - molecular biology - genetics - observed change |
|
|
|
What are the two processes that generate genetic variation?
|
- mutation
- sexual recombination |
|
|
|
In humans, what is the rate of mutation on a genetic level?
|
100k-200k replications of genes
|
|
|
|
What are 4 major areas in which biotechnology has applications?
|
- health care
- agriculture - industrial uses of crops and other products - environmental uses. |
|
|
|
Natural selection is the...
|
differential success rate in reproduction, and its result is adaptation
|
|
|
|
Heredity is the ...
|
continuity of traits from one generation to the next
|
|
|
|
The field of modern biotechnology is thought to have largely begun when? And why?
|
1980;
- US Supreme court ruled that a genetically-modified microorganism could be patented. - it involved a case of a bacteria that was developed that could break down crude oil spilled by oil tankers. |
|
|
|
What are 3 terms used that apply to the direct maniupulation of an organism's genes?
|
- genetic engineering
- recombinant DNA tech. - gene splicing |
|
|
|
In an ecosystem, what are 4 cycles that fix elements into a usable form?
|
- the water cycle
- carbon cycle - nitrogen cycle - phosphorus cycle |
|
|
|
Describe the biochemical processes of the water cycle:
|
- 2% of all water is fixed & held in ice or the bodies of organisms
- water is recycled through the processes of evaporation and precipitation |
|
|
|
Describe the biogeochemical process/implications of the carbon cycle
|
- 10 % of all available carbon in the air is fixed by photosynthesis
- plants fix carbon to form glucose, which is eaten by animals, who release CO2 through respiration, which the plants, in turn, fix. |
|
|
|
Describe the biochemical processes/implications of the nitrogen cycle.
|
- 80% of the atmosphere is in the form of nitrogen gas.
- Only a few genera of bacteria have enzymes that can fix nitrogen from gaseous form. - these bacteria live in the roots of legumes - nitrogen is necessary to make amino acids and the nitrogenous bases of DNA |
|
|
|
Describe the biochemical processes/implications of the phosphorous cycle.
|
- exists as a mineral
- fungi and plant roots have mycorrhizae that are able to fix insoluble phosphates into useable phosphorous. - phosphorous is needed for the backbone of DNA and for the manufacture of ATP. |
|
|
|
All acids contain...
|
hydrogen
|
|
|
|
Acid rain forms predominantly from ______ ______ in the air (usually ___ or ___ based ), which become _____ into their ______.
|
pollutant oxides; nitrogen or sulfer based; hydrated
|
|
|
|
Radioactivity is the...
|
breaking down of atomic nuclei by releasing particles or electromagnet radiation.
|
|
|
|
Radioactive nuclei give off radiation in the form of...
|
streams of particles or energy
|
|
|
|
Alpha particles are...
|
positively charged particles consisting of 2 protons and 2 neutrons
|
|
|
|
Beta particles are...
|
electrons
|
|
|
|
Beta particles are produced when... (3 points)
|
- a neutron in the nucleus breaks up into a proton and an electron.
- the proton remains inside the nucleus, increasing the atomic number by one. - but the electron is given off. |
|
|
|
Name 5 characteristics of gamma rays:
|
- are electromagnetic waves
- have extremely short wavelengths - have no mass - have no charge - not deflected by an electric field |
|
|
|
What does radioactivity do to the air it travels through?
|
it ionizes it
|
|
|
|
What are Darwin's 4 principles?
|
- individuals in a certain species vary from generation to generation
- some of the variations are determined by the genetic makeup of the species - more individuals are produced than wills survive - some traits allow for better survival of an animal |
|
|
|
What is a community?
|
a group of populations residing in the same area.
|
|
|
|
What are biomes?
|
communities that are ecologically similar in regards to temperature, rainfall, and the species that live there.
|
|
|
|
Name 8 biomes
|
- marine
- tropical rain forest - savanna - desert - temperate deciduous forest - Taiga - Tundra - polar or permafrost |
|
|
|
What is succession?
|
is an orderly process of replacing a community that has been damaged or beginning one where no life previously existed.
|
|
|
|
What are two types of succession?
|
- Primary
- Secondary - Climax |
|
|
|
What is primary succession?
|
occurs after a community has been totally wiped out by a natural disaster or where life never existed before
|
|
|
|
What is secondary succession?
|
takes place in communnities that were once flourishing but were disturbed by some source, by were not totally stripped.
|
|
|
|
What is a climax community?
|
a community that is established and flourishing
|
|
|
|
Name 5 feeding relationships:
|
- parasitism
- commensalism - mutualism (symbiosis) - competition - predation |
|
|
|
What is commensalism?
|
two species that occupy a similar place; neither species is harmed or benefits from the relationship.
|
|
|
|
What is carrying capacity?
|
- the total amount of life a habitat can support.
- carrying capacity decreases as the habitat runs out of food, water, shelter, or space. - stabilizes as life dies off |
|
|
|
What is biological magnification?
|
Arises when chemicals and pesticides accumulate along the food chain.
-tertiary consumers (e.g. humans) have more accumulated toxins |
|
|
|
What are 3 major crops that feed the world?
|
- rice
- corn - wheat |
|
|
|
What are biotic factors? (2 points)
|
- refer to living things in an ecosystem.
- if one population in a community increases, it affects the ability of another population to succeed by limiting resources. |
|
|
|
What are abiotic factors?
|
are non-living aspects of an ecosystem that, if changed, can result in the limitation or accelerating the growth of populations.
|
|
|
|
The carrying capacity of the environment is limited by... (2 points)
|
- the available abiotic and biotic resources (limiting factors)
- as well as the ability of ecosystems to recycle the residue of dead organisms through the activities of bacteria and fungi. |
|
|
|
Give 3 examples of abiotic factors.
|
- soil quality
- rainfall - temperature |
|
|
|
Give 4 examples of biotic factors:
|
- plants
- animals - bacteria - fungi, etc. |
|
|
|
What is homeostasis?
|
- it's the result of regulatory mechanisms that help maintain an organism's internal environment within tolerable limits.
|
|
|
|
What are the 3 homeostatic systems?
|
- osmoregulation
- excretion - thermoregulation |
|
|
|
What is osmoregulation?
|
deals with the maintenance of the appropriate level of water and salts in body fluids for optimum cellular functions.
|
|
|
|
What is excretion (in terms of a homeostatic system)
|
- the elimination of metabolic waste products from the body including water
|
|
|
|
What is thermoregulation?
|
- maintains the internal, or core body temperature of the organism within a tolerable range for metabolic and cellular processes.
e.g. vasoconstriction/dilaton |
|
|
|
In terms of ecology, what is biogeochemical cycling?
|
- it's the movement of chemicals between the biotic (living) and abiotic (non-living) parts of an ecosystem.
|
|
|
|
What are some examples of biogeochemical cycling?
|
- Respiration
- photosynthesis |
|
|
|
What are the 7 steps of the scientific method?
|
1. pose a question
2. form a hypothesis 3. Doing the test 4. Observe and record the data 5. Drawing a conclusion 6. Gathering data 7. Write report. |
|
|
|
In terms of the scientific method, the testing step should include what to make it good & fair? (3 points)
|
- must have a variable or any condition that can be changed.
- have a control group - a good test will try to manipulate as few variables as possible so as to see which variable is responsible for the result. |
|
|
|
In terms of the scientific method, the observation and recording step should:
|
- state the specifics of how the measurements were calculated.
|
|
|
|
In terms of the scientific method, the step of drawing a conclusion includes doing what? (2 points)
|
- Comparing data with that of other groups.
- a conclusion is the judgement derived from the data results. |
|
|
|
In terms of the scientific method, what should the written report include? (7)
|
- title
- abstract - defined purpose, includes hypothesis - description of what was done to to prove or disprove a hypothesis. - supporting data - observatons - conclusions |
|
|
|
In terms of the scientific method, between observation and experimentation, there are 3 important steps:
|
- gathering information (researching about the problem)
- hypothesis - designing the experiment |
|
|
|
In designing an experiment is important since it involves identifying the:
|
- control
- constant - independent variables - dependent variable |
|
|
|
In a scientific experiment, the control is:
|
factors you have to keep constant to get reliable results
|
|
|
|
In an experiment, independent variables are:
|
factors we change in an experiment
|
|
|
|
In terms of variables and constants, it's very important that:
|
there should be more constants than variables to obtain reproducible results
|
|
|
|
prefix for 10X the base unit:
|
deca
|
|
|
|
prefix for 100X the base unit
|
hecto- (meter, liter, gram)
|
|
|
|
prefix for 1000X the base unit
|
kilo- (meter, liter, gram)
|
|
|
|
prefix for 1/10 the base unit
|
deci-
|
|
|
|
prefix for 1/100 the base unit
|
centi-
|
|
|
|
prefix for 1/1000 the base unit
|
milli-
|
|
|
|
x-axis on a graph represents the:
|
independent variable (e.g. time): a variable that changes
|
|
|
|
y-axis on a graph represents the:
|
dependent variable (e.g. height): changes dependent on independent variable changes
|
|
|
|
What is chromatography?
|
uses the principles of capillary action to separate substances, such as plant pigments moving up paper.
|
|
|
|
What is spectrophotometry?
|
uses percent light absorbance to measure color change, thus giving qualitative data a quantitative value.
|
|
|
|
Centrifugation does what?
|
separates denser mass from lesser dense mass
|
|
|
|
What is electrophoresis?
|
uses electric charges of molecules to separate them according to size. e.g. DNA or proteins are separated in a gel box that has terminal ends
|
|
|
|
Physics is characterized by what? To do what?
|
by the use of mathematical equations to understand phenomena in a fundamental and ultimate manner.
|
|
|
|
Define the difference of studies between chemistry and physics:
|
chemistry studies: composition, structure, and properties of matter and condensed matter
Physics studies macroscopic and microscopic physical properties of matter |
|
|
|
Newton's laws can be said to be what of currently accepted laws of motion?
|
approximations
|
|
|
|
Newtonian laws of motion have been replaced with ______.
|
relativity
|
|
|
|
What culture is said to have first use the scientific method? About when?
|
Muslim scientists; middle ages
|
|
|
|
What western scientist is the earliest advocate of the scientific method? What century?
|
Roger Bacon (1224-1294)
|
|
|
|
When did the Scientific Revolution begin? And with what and who's theory?
|
16th century; Nicolaus Copernicus's heliocentric theory
|
|
|
|
When and who discovered that planets orbit the sun?
|
1605, Johannes Kepler
|
|
|
|
When did science begin as a profession and an institution in Western nation-states?
|
19th century
|
|
|
|
Science began with historical movement and when? And why is this considered to be the beginning?
|
the agricultural revolution; approx. 8000 B.C.
- there was apparently a body of knowledge that enabled humans to increase production. |
|

