![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
453 Cards in this Set
- Front
- Back
|
1. Risk of UTI increases with?
2. More then ___ of women will have a UTI? 3. UTI in men tend to be in: |
1. Age
2. 50% 3. Young Boys, Elderly, MSM |
|
|
What are some host defences against UTIs?
|
1. High volume urinary flow -- "flushing".
2. Low residual volume 3. Protective Components: Immunoglobulin, Urea Secretion of Lewis blood group antigens. |
|
|
Describe the normal flora of the urinary tract region.
|
1. Bladder and Kidneys are usually sterile.
2. Distal urethra colonised by a range of bacteria which includes: - Coagulase Negative Staphylococci - Diphtheroids - Non-pathogenic Neisseria sp. - Anaerobic Cocci - Mycobacteria - Mycoplasmas - Lactobacilli - -> Gram Positive Rods which form lactic acid (metabolic by-product) -> Strongly protective role, linked to their production of bacteriocins, hydrogen peroxide and acid (maintains acidic vaginal pH) -> Also presents on mucosal membranes of mouth and gut. |
|
|
How does the bacteria invade the urinary tract?
|
Invading bacteria must overcome flushing effects of urinary voiding. Colonisation of periurethral and sometimes rectal regions.
Bacterial ascent from urethra into bladder occurs with some species. -> Facilitated by placement of urinary catheter. Infection of otherwise healthy individuals requires expression of range of virlance factors such as : - Attachment Pili - Ig Proteases - Ureases |
|
|
1. UTIs are predominantly _______.
2. Which fungus is important in UTIs? 3. Some viruses are shed from the urinary tract w/out many symptoms, what are they? |
1. Bacterial.
2. Candida Albicans (Yeast form) 3. CMV and Rubella are shed in urine of congenitally infected infants. |
|
|
What are the two most important pathogens in UTI?
What other pathogens may be present? |
1.
#1 is E.Coli and causes 80-85% of all cases of acute cystitis. #2 S. Saprophytcus (GBS) 2. Others are: - Enterobacteriaceae, e.g. P. Mirabilis - Pseudomonas Aeruginoa - Proteus Mirabilis - Staph. Aureus, Staph. Epidermidis - Enterococcus sp. (E. faecium and E. faecalis). - M. Tuberculosis |
|
|
What is the difference between Complicated and Uncomplicated UTI?
|
1. Complicated UTI:
- There is obstacles to flow (increase in the volume of fluid retained) - Higher likelihood of developing urosepsis and renal damage. - Higher mortality rate - Reduced susceptibility to antimcrobials. Uncomplicated: - Absence of obstacles. - Susceptible to antimicrobial treatment. |
|
|
1.
Describe Complicated UTIs: 2. -> it occurs in individual with structural or functional abnormalities which maybe ? |
Symptoms are consistent with cystitis or polynephritis, depending on the level of involvement.
2. - Urinary flow obstruction - Kidney Stones - Biofilm - Urinary reflux - Catheters |
|
|
What is the difference between ascending and descending UTIs?
|
Ascending UTI: Bacteria travel from urethra to bladder and from there may also move to kidney.
Descending (less common): Microorganism is transferred to the kidney via haemotogenous (blood) spread. |
|
|
What are the key/common pathogens which are associated with Hospital Acquired Urinary Tract Infections.
|
E. Coli - 40%
Coagulase Negative Staphylococci (CNS) - 3% Other Gram +ve - 16% Candida - 5% P. Mirabilis - 11% Other Gram Negatives - 25% |
|
|
What is an important predisposing factor in HA-UTIs?
|
1. Catheterisation:
- enables direct microbial entery - Move up via either outside of catheter or inside, via lumen - Risk of UTI increases by 3-5% for each day of cathetaraisation. |
|
|
What is the role of biofilm in terms of UTIs.
|
- Protective being in the context of predominant form of growth for normal microflora.
- Problematic because it can be a reservoir for potential pathogens. - Colonization of the catheter can rapidly occur, especially with urinary catheters in place for an extended period of time. - Catheter in place for 7 days causes an infection in 10-50% of the patients. - Catheters in place for more then 28 days will cause an infection in ALL patients. ** Catheter associated UTis are the most common complication associated with catheterization. ** |
|
|
What are the main symptoms of UTIs?
Define the symptoms and Charachtaristics associated with: i. Cystitis ii. Pyelonephritis ii. Prostatitis |
1. ~50% of UTIs produce NO recognizable symptoms.
2. i. Cystitis [ Symptoms] - Dysuria (Pain Urinating) - Frequency - Urgency - Suprapubic Pain and Tenderness Cystitis [Charachtaristics]: - Acute Onset - Increased symptoms to severity relative to urethritis - bacteruria - hematuria Pyelonephritis [Symptoms]: - Flank Pain - Fever of > 38.3 C. - Similar symtoms as for cystitis Prostatitis [Symptoms]: - Pain in lower back, perirectal area and testicles. - High Fever - Chills - Symptoms of cystitis Prostatitis [ Characteristics ]: - Acute: usually in young men. Symptoms of acute infection maybe absent in chronic infections. |
|
|
What is Acute Cystitis:
|
It is the most common UTI presentation. Shows as:
- Dysuria "Gotta Go" - Frequency [increased] - Urgency - Painful Voiding Very in intensity: mild to sever from patient to patient. |
|
|
What are the members of Enterobacteriaceae and what are their shared/common features:
|
1. The members include. E.Coli, Morganella Moranii, K. pneumoniae, Proteus Mirabilis.
Common Features that they share are: - Gram Negative (LPS) - Rods - Normal Habitat: GI tract of humans and animals. - Primarily cause opportunistic infections such as UTIs - Enter and attach to uroepothelial cells. - Some are capable of further spread to bladder and kidney. |
|
|
E.Coli (UPEC, Uropathogenic E.Coli)
Gram Negative Rod - How does it enter? - What role does adhesion play, how does it adheres? |
- Entry via minor damage to uroepithelium (i.e. sexual intercourse). Often spread is from perineum.
- Adhesion plays a key role in establishment of an infection. 2 Classes of Adhesins: - Fimbrial (Type I and P pili) - infection of bladder and kidney (pyelonephritis) respectively. - They mediate invasion, resultin in persistence and intracellular biofilm like communities ( IBCs ). - Key contributor to recurrent infections. - P Pilus is anchored to outer membrane; distal end contains adhesin. - Bind to receptor located on surace of bladder mucosal strains - High incidence of expression in strains associated with pyelonephritis: > 90 %. - Expression in strains causing lower UTIs: ~ 30%. Type I Pilus: expressed more in strains associated with cystitis. - Important in initial colonisation of perurethral region. - Phase Variable - Bind to major glycoproteins of uroepithelial surface. Second Type: Adimbrial - various surface components and structures. |
|
|
What are some virulance factors in E.Coli?
|
1. alpha-haemolysin: channel-forming cytotoxin.
2. Siderphores: Aerobactin 3. Pathogenicity Islands |
|
|
What are some virulence factors of Proteus Mirabilis? What are some characteristics of it?
|
Its is gram negative and it is motile. It forms swarming colonies on solid media.
Virulance Factors are: - Proteases - Haemolysins - Biofilm formation - Production of bacterial urease produces alkaline enviornment via production of ammonia -> elevates local pH. -> Miniral precipitation can occur and deposit in biofilm, contributing to rapid blocking of the catheter. -> Also observed with: P. vulgaris, P. aeruginosa and K. Pneumoniae. |
|
|
Describe Staohylococcus Saprophyticus?
|
1. 5 - 15% of remaining cases of acute cystitis is not due to E.Coli.
2. -It is a gram positive coccus -Group B Streptococci (GBS): -Pyogenic Group (16s rRNA) -Coagulase Negative -Mostly Opportunistic Pathogens -> Important cause of neonatal meningitis: transfered from moher to baby during birth. Virulance Factors: EPS production and biofilm formation. |
|
|
Describe some other Urinary tract clinical syndromes:
|
- Urethral Syndrome
- Renal Abscess ---> Haematogenous: usually due to S. Aureus, can also be due to mixed aerobes and anaerobes. - Acute Prostatis --> Same microbial causes for acute cystitis --> E.Colis --> Other Enterobacteriaceae --> Enterococcus Species. --> Neisseria Gonorrhoeae -Renal Caliculi (Kidney Stones): --> Proteus Species, due to urease production and conversion of urea to ammonia -> alkaline urine). --> Morganella Morganii --> K. Pneumoniae --> Corynebacterium Urealyticum --> S. Saprophyticus --> Ureplasma Urealyticum |
|
|
Bacterial Counts in UTI can range from _____ to _____ so you need to consider?
|
10^2 to 10^6. Need co consider combination of pathogens (on lab detection) plus symptoms, which is considered diagnostic.
|
|
|
What is the common media used for UTI bacteria?
|
- Blood Agar (Enriched)
- MacConkey's Selective [bile and salt] and differential .(used to differentiate between bacteria that can and cannon produce lactose) -> Lactose vs. non-lactose fermenters (acid production will give a color change). |
|
|
How do you measure pyuria (presence of pus in the urine)?
|
- Microscopy: > 10 white cells per mm^3 of urine.
- Dipstick Leukocyte esterase (production of inflammatory cells) test. Treatment: - Requires specific, directed therapy. ----> Need to take into account bacterial resistance Preferred: Compounds that are excreted in high concentration in the urine. ---> for polynephritis need also adequete levels in tissues. Asymptomatic Bacteruria - usually not treated (EXCEPTION: PREGNANT WOMEN). - 30-50% of pregnant women w/ acute polyonephritis give birth prematurely. |
|
|
List the Top 10 STIs?
|
1. Papillomavirus
2. a. C. Trachomatis (D-K serotypes) b. C trachomatis (L1, L2, L3 serotypes) - lymphogranuloma venereum 3. Candida albicans 4. Trichomonas Vaginalis 5. HSV type I and II 6. Neisseria Gonorrhoeae 7. HIV 8. Treponema Pallidum 9. HBV 10. Haemophilus Ducreyi |
|
|
Which pathogens do you report to the CDC?
|
- Chancroid (H. Ducreyi)
- C. trachomatis - Gonorrhoea - HIV - HBV - Syphillis |
|
|
Most STIs cause some sort of lesion at the site of entry, what are the exceptions?
|
HIV and HBV
|
|
|
What is the normal site of entry for STI?
|
Mucosal/Squamous Epithelial layers:
- Cervix, Urethra, Rectum, oral Pharynx, Vagina. |
|
|
Do STI pathogens have animal or enviornmental reservoir? what is the primary reservoir?
|
They usually do not, the primary reservoir is asymptomatic carriers.
|
|
|
Increase in many STIs are on an increase, what is the contributing factor?
|
- Increasing Human Population which is densely located in cities and urban centers
- Difficulty of causing changes in sexual behavior - Absence of vaccines (exception is HPV, HBV). - Complex Infections: microbial and host components, behavioral and social factors. |
|
|
Certain STIs can progress to chronic infections such as:
These recurrent infections then may cause? |
- Pelvic Inflammatory Disease (PID)
- Anogenital Cancer - Secondary and Tertiary Syphilis - Recurrent Herpes Infection The recurrent infections may cause: -> Fallopian Tube Scarring -> Ectopic Pregnancy -> Congenital Diseases -> Increased Risk of acquiring HIV --> 3-5 fold increase risk, in presence of both ulcerative genital disease, non-ulcerative disease or certain vaginal diseases. (e.g. bacterial vaginosis). - Adverse outcomes of pregnancy (premature labour, stillbirth, etc). |
|
|
Transmission to STIs to kids can occur before or during birth. What do these cause in infants?
- HSV - C. Trachomatis - T. Pallidum |
HSV - > Herpes Simplex Virus
C. Trachomatis -> Trachoma T. Pallidum -> Congenital Syphilis |
|
|
HIV increases the risk associated with which disease?
|
1. Gonorrhoea and Chlamydia
|
|
|
List the diseases both viral and bacterial which are transmitted primarily via sexual activity:
|
Viruses:
HPV HSV CMV HIV HTLV-1 HCV HBV(HDV) Bacteria N. Gonorrhoeae H. Ducreyi C. Trachomatis T. Pallidum |
|
|
What are the advantages to the pathogen for the Sexual Transmitted Route:
|
- No vector or vehicle necessery
- No adaptations necessary to enable long-term survivial outside of the human body - Infected individuals are usually otherwise healthy, young and active - No Dorman or Latent State necessary (but may be part of the pathogenesis). - Don't have to be highly virulent |
|
|
How do STI generally present in men and women?
|
Man: Urethritis, Genital Ulcers, Genital Warts
Women: Cervicitis, Vaginitis |
|
|
What is Urethritis?
|
It is an infection of the urethra (male and female)
Key Bacterial Agent is: N. Gonorrhoeae, C. Trachomatis, Ureaplasma Urealyticum (can be cause of nongonococcal urethritis in men). |
|
|
Describe Niesseria Gonorrhoeae:
|
Other names: "clap", "the drip"
Obligate Human Pathogen, No Animal or Enviornmental Reservoir Gram Negative Diplococci "Coffee Bean" --> Characteristically intracellular in polymorphonuclear cells of inflammatory exudates. |
|
|
What is the primary site of infection of N.Gonorrhoeae in men and women?
|
men:
- usually symptomatic, - copious yellowish pus - Pain on urination Cervix ("Cervicitis"): - Preferential attachment to columnar epithelium - Symptomatic in <50% of women. ---> Cervical Discharge, Vaginal Bleeding, Abdominal Pain ---> Asymptomatic Carrier State important for maintain of infection in the population. |
|
|
What can be a complicated infection of N. Gonorrhoeae?
|
PID, Salpingitis, more likely to occur in women.
Disseminated Infection can occur: -> Pustural Exanthema -> Tendon and/or joint inflammation-> supprative arhritis -> Fever |
|
|
What is the Pathogenesis of N.Gonorrhea:
List the methods of attachment |
Attachment occurs to columnar epithelium of distal urethra or cervix via:
-> Pili -> Outer membrane/surface proteins (Opa) -> Por Protein -> Lipooligosaccharide (modified version of LPS) -> Iron Binding Proteins --------------------- Interaction between bacterial and host cell triggers inflammatory response. Cell Invasion occurs, facilitated by bacterial Por (Outer membrane porin). -> Prevention of phagosome-lysosome fusion. Large Number of Genetic Variants = repeated infections, with no protective immunity. Produce IgA protease. Chronic Infections can cause stricture and scarring of either fallopian tubes or urethra. |
|
|
How do you diagnose N.Gonorrhoeae?
|
Microscopic examination of mears for intracellular Gram Negative Diplococci:
-> From symptomatic men: > 95% sensitivity -> Women or Asymptomatic Men: 30-50% sensitivity. Isolation on appropriate media requires for confirmation. -> Fastidious: Highly Sensitive to drying and temperature changes. -> Incubate in high humidity (>90%) and increased CO2 (5-10%). -> Grows on CHOCOLATE agar --> Usual lab medium for isolation if other bacteria is present. Thayer Martin [VCN Agar] = Vancomycin, Colistin and Nystatin. - if resulting colonies are Oxidase Positive (Cytochrome enzyme) = Presumptive ID of Neisseria is possible. |
|
|
What is important in successful control of N. Gonorrhoeae?
|
- Effective Antibiotics
- Rapid Diagnosis - Prevention of Spread - Contact Tracing, Examination and Treatment. |
|
|
Describe the antibiotic resistance of N.Gonorrhoeae?
|
1. Chromosomically encoded: mutation in genes for efflux pump (resulting in its over-expression): mutation in por, mutation in PBP1 (affecting penecillin binding)
2. Plasmid Encoded: - Enzyme Mediated: Penicillinase (TEM B-Lactamase) - PPNG: Penicillinase Producing Neisseria Gonorrhoeae. |
|
|
in N. Gonohhrea why is the Fluroquinolone (Ciprofloxacin) resistance inceasing?
|
Its Chromosomally Mediated: Mutation in the DNA gyrase.
|
|
|
In N. Gonorrhoeae, the topoisomerase IV gene cause limit the use of what as a treatment option?
|
1. Cipro
|
|
|
Describe Chlamydia Trachomatis?
|
It is an atypical bacteria, small obligate intracellular parasites.
|
|
|
Which serotypes are associated with STIs?
|
The serotypes associated with disease are D-K
|
|
|
Which are the syndromes cause in MEN by Chlamydia?
|
Urethritis
Epididymitis Proctitis |
|
|
Which are the syndromes caused in WOMEN by Chlamydia?
|
Urethritis
Cervicitis Bartholinitis Salpingitis |
|
|
What is the most common sexually transmitted disease in the civilized/industrial world?
|
1. Chlamydia
-> 4 million new cases per year in the USA. -> High percentage of infections in women are subclinical: 50-70% Majority of infections: 15-24 years of age. |
|
|
What are the 2 types of Chlamydial Forms?
|
Elementary Body: Extracellular Form ~ 0.2 um. Enters the host via RME, Modifies endocytic vesicles, no active metabolism. *infectious form*
Reticulate Body: Intracellular form, Larger then 0.8 um. It cannot survive outside of the host cell. Metabolically active. |
|
|
How do you diagnose Chlamydia?
What is the GOLD STANDARD? |
1. Challenging because multiple infections are common:
--> Highly Likely: Gonorrhoea plus Chlamydia infections GOLD Standard: Isolation and Culture Microscopic Examination for INCLUSION BODIES Newer Tests: Ur-Sure PLUS test Urine, male urethral swab or female endocervical swab. For Screening only. |
|
|
Describe Ureaplasma Urealyticum?
|
- Member of Class Mollicutes ("Soft Skin")
- Closely Related to Mycoplasma Sp. Is the only species in its class. - Highly Variable Morphology - Spherical Throught ccoccoidal to short and branching. Cholesterol is an important component of cell membrane. It is named like this because it can convert urea to ammonia (urease) |
|
|
Describe how Chlamydia goes from Endosome to Elemntry body?
|
A. The infectious particle is the Elementry body. The EB attaches and enters via REM. into the columnar epithelial cells that line the mucus membrane.
Once in the endosome, the EB inhibits phagosome-lysosome fusion, and is not destroyed. It then transforms into an initial body (IB). Once you have a lot of IB, some transform back into EB, which can now effect more cells. |
|
|
Which microorganisms cause Genital Ulcers?
|
Associated Microoganisms:
i. T. Pallidum ii. H. Ducreyi iii. C. Trachomatis iv. Klebsiella Granulomatis v. M. Tuberculosis vi. Herpes Simplex Virus |
|
|
Which organism causes Syphillis?
|
Treponema Palliudm
Synonems: "The Great Pox" |
|
|
What are the three stages of Syphilis?
|
1. Primary:
Highly Infectious Painless (but sensitive) ulcer 9-90 days post-exposure -> papule. Secondary: 2-8 weeks after ulcer. Generalised maculopapular rash & multiple symptoms indicative of systemic infection - Rush on palms and soles, joints, 1/3rd of patients: condylomata latum (painless warty lesions). Latent Syphilis: - 25% may relapse and develop secondary stage symptoms again. Tertiary Syphillis (not everyone gets this): Often after 15-20 years post-initial infection: Focal Lesions: Sites vary. Most Important are: -> Cardiovascular (aortic aneurysm) -> Neurosyphyllis, damage to CNS-- progressive dementia; meningitis; hallucinations, etc. Good Summary on p.127 of Microbiology Made Simple |
|
|
Describe the microbial characteristics of Syphillis?
Can you culture it on artificial media? |
1. Spirochette, 0.2 um Diameter, 5-15 um lenght
It is an obligate human pathogen: unculturable on artificial media. Unusual outer membrane: no LPS, no porins. Lipid-Rich Surface Layer, located in periplasmic space. Endoflagella. |
|
|
What is the Epidemiology of Syphillis?
|
Significant public health problem worldwide. Estimated 12 million new cases per year.
Transmission is exclusively by direct contact with primary or secondary syphilitic lesions. |
|
|
How is Syphilis transmitted?
|
Direct contact with primary or secondary syphilitic lesions.
|
|
|
What is the pathogenesis of syphillis?
|
1. Primary Syphillis:
-> Enters into subepithelial tissues via breaches in skin barrier -> Slow multiplication doesn't cause overt tissue damage. -> Produces endarteritis and granulomas -> Primary Lesions heal but bacteria disseminate via lymph nodes and blood stream. Mechanisms controlling latency not understood. Secondary: - Disseminated Infections. - Evasions of immune system occurs but mechanisms are unclear - Contribution of lipid surface layer in enabling evasion of immune response is suggested. - Contribution of inflammatory response may be responsible for some of the observed symptoms. Tertiary: - Delayed-Type Hypersensitivity Reaction. to persistent spirochetes probably contributes to tissue damage. |
|
|
Describe the Diagnosis of syphilis?
|
1. You can do a microscopic test using darkfeild microscopy. This can reveal tiny helically-shaped organisms moving in a corkscrew-like fashion.
Presumptive, can measure active, primary syphillis. Highly sensitive to environmental changes: drying temperature elevations, etc. 2. Serological Tests: - Infection results in cellular damage, causing to release lipids, including cardiolipin, and lecithin. Body will produce antibodies against these antigens. Measure the antibodies, if the patient's serum has these antibodies, syphilis is suspected. Two most common tests employed are: Venereal Disease Research Laboratory (VDRL); MICROSCOPIC and Rapid Plasma Reagin (RPR) test: MACROSCOPIC Mixture of VDRL and Plasma: Flocculation if antibody is present. |
|
|
Describe the Confirmatory Test for Syphilis?
|
Confirmatory:
All positive screening tests are confirmed due to false positives. Utilise Specific Treponemal Antigen and EIA: - Detect IgG and/or IgM TESTS: - TPPA (T.pallidum particle assay) ---> Specific Antigen attached to gelatine beads - TPHA (T. Pallidum haemagglutination assay) ----> Specific antigen attached to erythrocytes - FTA-ABS Flurescen-treponemal antibody-absorbed trest - MHA-TP. Microhaemagglutination assay for T.Pallidum. |
|
|
For HIV positive patients, during syphilis diagnosis what must be done?
|
- Need to dilute patient's serum in order to get a positive reaction. This is because in HIV, Cardiolipin levels are elevated.
|
|
|
What causes the STI chancroid?
|
Haemophilus Ducreyi.
|
|
|
How does chancroid present clinically?
|
It present with painful genital ulcers. Unilateral painful swollen inguinal lymph nodes rapidly develop in half of the infected persons. The lymph nodes become matted and will rapture, releasing pus.
Entry of organisms is via small epidermal surface abrasions. Location of ulcers: Men: prepuce, frenulum Women: Vulva, Cervix, Perianal Region. Predominant in the tropics, occasionally in North America and Europe. |
|
|
How do you diagnose Chancroid?
|
Cultivation of this bacterium is difficult.
Highly Specialized medium is required (organism is fastidious) Culture Medium Options: -> GC based with 1% haemoglobin, 5% fetal calf serum and 1% IsoVitaleX -> Mueller-Hinton agar w/ 5% chocholatised horse blood and 1% IsoVitaleX. 5% CO2 enriched atmosphere; incubation at 33C for 48-72 hours. Increasing antibiotics resistance due to resistance plasmids and chromosomal mutations. |
|
|
Describe the Herpes Simplex Virus?
|
Herpesviridae: Alphanerpesvirinae
Linear dsDNA Enveloped: Projecting Viral Glycoprotein Spikes Between envelope and capsid: tegument: contains virally-encoded enzymes & transcription factors. Neurotropic. Replicate in skin or mucosal epithelial cells --> localised vesicular, mucocutaneous genital lesions: -> Fever -> Malaise -> Regional Lymphadenopathy -> Urethritis -> Ulcers/Lesions HSV transmission: -> HSV type I : oral-oral; oral-genital -> HSV type II: primary genital-genital (other routes as for HSV-1 also possible) -> Infected Individuals (primary or reactivations) can be shedding the virus from epithelial cells w/out obvious lesions ("asymptomatic shedding"). Majority of infected individuals are thought to be asymptomatic. |
|
|
What is the Pathogenesis of Herpes Simplex Virus?
|
Site of Entry: Mucous Membranes, skin
Exposure is via intimate sexual contact (auto-inoculation also possible) |
|
|
How does the Herpes virus gets replicated/synthesized?
|
1. Immediate Early (IE): proteins to start & regulate viral transcription
2. Early (E): viral enzymes for genome replication (DNA polymerase, helicase, thymidine kinase). 3. Late (L) stage - 12- 15 hours post-infection; mostly structural proteins, proteins for viral assembly, etc. One of tegument proteins induces cellular RNase --> degradation of cellular mRNA and halting protein synthesis: --> productively infected epithelial cells are usually killed. 11 known HSV surface glycoproteins. Role in pathogenesis established for 3: -> gB and gD: attachment and entry -> gH: viral release Differences in gB account for the two antigenic types. In Active Infection: Viral Invasion and Replication -> Painful vesicle. In Primary Infection, can get more then 20 lesions. Collapse of Vesicles -> Ulcer Formation On skin, ulcer then crusts over. Localised lesion can result in nerve damage or inflammation -> Symptoms of: itching, burning, tingling or pain. Eventually damaged epithelial cells are regenerated. |
|
|
What is the Diagnosis for Herpes Simplex Virus:
|
Clinical Presentation plus patient history:
Scrapings of lesion and examine microscopically: look for multinucleate giant cells (Tzank Cells), w/ intranuclear eosionophilic inclusions. --> this is less sensitive then culturing, its not specific. Virus Detection: inoculation of vesicle fluid or or scraping into human diploid fibroblasts. CPEs visible in 24 hours. Complement test also possible but levels of antibodies can be low in genital infections EIA more sensitive. |
|
|
Describe Papillomavirus (HPV)
|
Family: Papovaviridae
dsDNA small: 55nm Nonenveloped Stable, Can survive on and be transmitted via fomites. |
|
|
What are the different types of warts in HPV?
|
1. Plantar
2. Flat warts of other skin areas 3. Laryngeal papillomas 4. Genital Epithelial Lesions --> Cervical, Vulvar, and penile warts and papillomas |
|
|
What does HPV infect?
|
Infects Squamous and Columnar Epithelium.
Virions present in upper most layer in terminally differentiated keratinocytes. --> E7 gene products bind to Rb, enabling continued growth and viral expression through prevention of keratinocyte differentiation. |
|
|
Describe the external genital infections: exophytic genital warts (condylomata acuminata) - HPV
|
Usually found in sexually active adults.
Women: vulva, vagina and on cervix Men: Penis shat, peri-anal skin, anal canal. |
|
|
Describe Genital HPV:
|
most often benign, with spontaneous reversion.
- HPV genotype 16: most commonly associated with malignant lesions & progression to cervical intraepithelial neoplasm -> dysplasia and/or carcinoma. |
|
|
How do you diagnose HPV?
|
Papanicolaou Smear:
-> Pokilocytosis: vaculated cells with enlarged nuclei (koilocytes). -> Not always present Histological Examination of Biopsy PCR for detection of specific viral DNA. |
|
|
Which microbes can caused Bacterial Vaginitis/Vaginosis (BV)
|
- Mobiluncus Sp. (M. Curtisii, M. Mulieris)
--> Anaerobic Curved Rods --> Gram Variable (M. Curtisii) and Gram Negative (M. Mulieris) on staining bur presence of thin cell wall means they should be considered as Gram Positive. - Gardenerella Vaginalis --> Microaerophilic: Gram Variable, Pleomorphic Rod. - Mycoplasma Homins --> Highly Variable Morphology. Changes in vaginal flora results in predominance of these species. --> Attach to squamous epithelial cells "clue cells" - Vaginal Fluid pH increases to > 5.0 (from <4.5). = main cause of increased vaginal discharge amongst women of childbearing age. "Rotten Fish" odour often reported. This is considered sexually associated rather then sexually transmitted. |
|
|
How do you diagnose Bacterial Vaginitis/Vaginosis (BV) ?
|
4 Criteria:
-> Clue Cells -> Amines: detection is via KOH to discharge (fishy smell becomes obvious, more pronounced on alkalization). -> Elevated Vaginal pH -> Thin Vaginal Discharge 3 or more of these = BV. ---- Alternative--- use staining of vaginal smears and subsequent grading of the flora: Grade 0: Normal: No Bacteria on Epithelium Grade I: Normal: lactobacillus morphology only. Grade II: Lower numbers of lactobacillus + mixed bacterial flora Grade III: mixed bacterial flora + few/absent lactobacillus morphotype Grade IV: Normal inasymptomatic women: epithelials with gram positive cocci + few/absent lactobacillus morphotype. Even with treatment, recurrence is common . |
|
|
What is opportunistic STIs?
|
Oral-Anal contact may result in sexual transmission of e.g. Salmonella, Shingella, Hepatitis A virus, Giardia Lablia, and Entamoeba Histolytica.
|
|
|
Who discovered HTLV-1 , HTLV -2 and HTLV-3(HIV)
|
- HTLV-1/2 is Robert Gallo
- HTLV-3 (HIV) Luc Montaginer |
|
|
HIV has what type of genetic information?
- What genes does it contain? - To be encoded, it requires what? - These viruses are? |
- RNA double stranded DIPLOID (dimeric). [diploid (+) ssRNA]
- env, pol and gag genes - RNA dependent DNA polymerase - SPECIES SPECIFIC |
|
|
THE HIV VIRUS:
the enzyme complex is composed of? - What is the group specific antigen? |
- RNA-Dependanted DNA polymerase/RNAae H
- GAG is the group specific antigen |
|
|
1. The HIV DNA replicates where? does it integrate?
2. HIV is a ______ Virus? 3. How can they be transmitted? |
1. Nucleus, yes it integrates.
2. Cytolytic 3. Body Fluids, in Utero, and some retroviruses can be transmitted by insect bites. |
|
|
Retroviruses have been associated with?
|
1. Oncogenesis, lymphoprolifirative disease, neurological disorders.
|
|
|
What is the subfamily of the important Retroviridate for our studies?
What are the important genus ? |
Orthoretrovirinae:
Genus: Alpharetrovirus -> Avian Leukosis Virus -> Rous Sarcoma Virus Genus: Deltaretrovirus: -> HTLV I -> HTLV II Genus: Lentivirus --> Human Immunodeficiency Virus contains major clades: A,B,C,D,F,O --> Human Immunodeficincy Virus II contains major clades A, B. --> Visna Virus Subfamily: Supmavirine: Genus: Spumavirus --> Simian Foamy Viruses Clades are subtypes of the virus that have evolved since the viruses emergence. The members share homologues features and share a common ancestor. Differ in amino acid content by at least 20% (env) and 15% (GaG) |
|
|
What are the major clades of HIV-1 in US, Africa and asia?
|
US: HIV-1B
Africa: HIV-1C Asia: HIV-1E |
|
|
Describe how the cross-section of an HIV virus looks?
|
- Enveloped Virus
- Two Identicle RNA strands - RNA polymerase - Integrase - two transfer RNA(tRNA) based-paired to the genome within the protein core - Surrounded by protein and lipid bilayer - The envelope spiked are the glycoportein are (gp) 120 attachment protein and gp41 fusion protein. |
|
|
Compare to HIV, HTLVII/II are what type of virus morphology?
|
- HIV is Type D particle
- HTLV I / II are Type C particles |
|
|
Describe the life-cycle of HIV virus?
|
- HIV binds to CD4 and chemokine co-receptors and enters by fusion.
- Genome is reverse transcribed - Gets integrated into the DNA - Translation/Transcription |
|
|
The production of the messenger RNA for HIV and HTLV-1, respectivly requires what?
|
HIV-1 - production of tat and rev
HTLV-1 tax and rex. They require the excision of 2 introns. |
|
|
HIV rev is required for what?
|
Viral Assembly
|
|
|
What are the two main disease of HTLV-1 and HTLV-2 ?
|
- Adult T-Cell Leukemia (ATL)
- HTLV-1 associated myelopathy/tripical spestic paraparesis (HAM/TSP) HTML-associated uveitis and dermatits are also associated with HTLV-1. |
|
|
If a patient has a high antibody titer for HTML-1 in their serum or CSF, what disease may he/she have?
|
- Tropical Spastic Paraparesis (TSP)
|
|
|
Patients with chronic myelopathy and recognition of small population of adult T-cell leukemia (ATL)-like cells in CSF/blood would indicate?
|
HAM = HTLV-Associated Mylopathy
|
|
|
1. Unlike HIV there is a ____ homology between HTLV-1 and HTLV-2?
2. HTLV-1 has ___ potential? 3. HTLV-1 is transmitted in three ways: 2. |
1. 96-99%
2. Oncogenic 3. i. Mother to child ii. Sexually, mainly male to female iii. from blood and blood products. |
|
|
What is the mechanisms of retroviral oncogenesis?
|
1. The oncovirinae, RNA tumors are known to cause: leukemias, sarcomas, and lymphomas.
2. These viruses are not cytolytic 3. Transformation results from the overproduction or altered activity of the growth-stimulating oncogenic product. 4. May need helper viruses for replication 5. May remain endogenous and transmit vertically via germ line of the animal. |
|
|
1. Where can you find HTLV-1?
2. How is does it infect? |
1. Japan, Taiwan, West Indies and Northeastern South America, Central America and Central Arica.
2. Affects CD-4 T cells and Delayed Hypersensitivty T-Cells. They tend to reside in the skin and contribute to symptoms of Adult-T-Cell Leukemia. - Neurons also can express receptors for HTLV-1 |
|
|
What is the epidimiology of HTLV-1
|
- occurs early in life
- usually females - varible sero-prevalance |
|
|
How do you diagnose HTLV-1
|
- Serology
- Can be done by ELISA and Agglutination - All screening require confirmation using Western Blot Techniques - To confirm the diagnosis of ATL (adult-t-cell leukemia), the provirus in leukemic |
|
|
Which virus causes Infection of the macrophages?
|
- Visna/Maedi Virus (most important)
- Equine Infectious Anemia look at page 37-38-6 for more. |
|
|
Which virus causes infection of the T-lymphocytes?
|
Feline/Simian and Human immunodeficiency viruses.
|
|
|
What is the origins HIV?
|
- Most evidence points to Africa
- Most believe it came from non-human primate species. - HIV-2 probobly came from the Sooty Mangabey in West Africa. Currently: - "Cut Hunter Theory" --> HIV came from animals --> humans - NO support the HIV came from a contaminated polio vaccine. |
|
|
What is the estimated infection of HIV end of 2003?
|
~ 46 million
- Highest Rates are in sub-Saharan Africa. |
|
|
Which category of people is the largest exposure category for HIV?
|
- Homosexuals
- Intravenous drug users and heterosexual parters is becoming more prevalent. |
|
|
What are the characteristics common to Lentiviruses: HIV-1 and HIV-2
|
Clinical Characteristics: Long Incubations, immune supressions, hematopoetic system, CNS, Arthritis.
Biological Charactariticis: Host species specific, exogenous and NON-ONCOGENEIC, CPE in certain infected cells, latent or persistent infection in some cells, cone shaped nucleoid (type D) TEM. Molecular Characteristics: Large Genome, Truncated GAG gene, several processed gag proteins, polymorphism in envelope region, novel central prf seprating the pol and env regions. |
|
|
What is the disease mechanisms of HIV?
|
- HIV primary infects CD4 T cells and cells of the macrophage.
- Virus causes lytic infection of CD4 T cells and persistent low-level productive infection of macrophage lineage cells. - Viruses causes syncytia formation, with cells expressing large amounts of CD4 antigen with subsequent lysis of the cell. - Virus alters T-cell and macrophage cell function. |
|
|
What are the pathogenic mechanisms of HIV-1 virus?
|
- Direct Killing of the cell (TAT enhanced)
- Dysfunction if infected immune cell - Aberrant production of cytokines - Immune mediated destruction of virus infected or antigen coated cells - Various other indirect mechanisms (i.e. ANERGY, apoptosis superantigen induced cells, prolifiration and depletion of immune cells, defective signaling, molecular mimicry and autoimmunity). - Direct or indirect action of non-structural regulatory genes (i.e. nef, tat and rev) |
|
|
HIV binds to the following molecules:
|
- CD4 antigen on helper T ells
- Co-receptor CCR5 (cemokine) ---> CCR5 Delalta 32 deletion mutation blocks entry -> Integrin alpha 4 beta 7 in the gut ---> helps spread HIV cell-to-cell. |
|
|
Infected macrophages (HIV) can do the following?
Destruction of CD4 t cels can cause what? |
- act as a reservoir for the infection
- transport agent to infect microglial cells in the brain, the pulmonary alveolar macrophages and the dendritic cells in the skin. - In CNS virus can cause AIDS dementia - Lysys of CD-4 T cells and DTH T cells causes: -- immunodeficiency -- loss of B-cell control -- lymphadenopathy -- hypergammaglobulinemia All of this can cause recurring infections such as Kaposi's Sarcoma and Lymphoma. |
|
|
What are ways HIV evades the immune system:
|
- inactivation of immune system
- eliminating the central controlling element of the immune system - loss of DTH T cells - antigenic drift of gp120 viral attachment protein |
|
|
What can predict the speed of HIV spread?
|
- Circulating viral load in serpositive patient's on their first visit.
-- Viral load is assyaed using RT-PCR - CD4/CD8 Ratios is also useful, used in conjunction with viral load. |
|
|
What is the clinical course of HIV?
|
- Long clinical latency period with mononucleosis like symptoms.
- The progressive decrease in CD-4 T cells allows for recurrent infections of occur. - The stages in HIV disease are defined by the CD4 T-cell levels and occurrence of opportunistic disease. |
|
|
What are the clinical symptoms of HIV?
|
- CD4 T cells below 100 -> Toxoplasmic Encepholitis
- Feer, nights sweats, fatigue, wasting syndrome (cahexia) chronic diarrhea - Aids dementia complex (ADC), apathy, loss of concentration, depression, disorientatio - Opportunistic Infection (CD 4 T below 73 = Cryptococal Manangitis) - Onset varies with CD4+ Count - Positive EIA (Immuno) and Western Blot - T-cell dysfunction - Viral load is better predictor of long term outcome then CD4 Counts. |
|
|
How can you protect the fetus from infection from an HIV positive mother?
|
- Use or prophylactic AZT (Azidothymidine) treatment of the mother.
- Zidovudine in labor and 6 weeks to the neonate - Cesarean delivery should be recommended to all women with viral load greater than 1000 copies. - Maternal antibodies from a positive mother can lead to a false positive for HIV in new born --- repeated testing needed to confirm infection. --- Congenital transfer of virus from the mother. |
|
|
Is HTLV-1 transmitted in utero?
|
Rarely, usually its breast milk.
|
|
|
How is HIV Diagnosed?
|
Detection of antibodies by ELISA screening
-- highly sensitive but false positives can occur Western blot confirmatory test -- Detects antibodies against HIV envelope and core proteins (60-90%) cross-reactivity with HIV-2 Detection of core p24 antigen by PCR Oral Tests Now Available |
|
|
The western blot for HIV detects?
|
- Serological Method p24 antigen of HIV
- Miagration studies using PAGE separating molecules based on weight. - Transferred Nitrocellulose gel and reacted with labeled serum - Can detect HIV, HTLV and Lyme disease and others. |
|
|
What is the best treatment method for HIV?
|
Prevention.
|
|
|
What are the various drugs used to fight HIV?
|
Sites to block HIV replication therapy of HIV infection:
- Nucleoside-Analog Reverse Transcriptase Inhibitors (NRTI) -- Zidovudine (AZT) -- Didanosine (ddl) -- Zalcitabine (ddC) -- Stavudine (d4T) -- Lamivudine (3TC) Epivir - Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) -- Nevirapine (Viramune) -- Delavirdine (Rescriptor) - Protease Inhibitors -- Saquaninavir (Inavir) -- Ritonavir (Norvir) -- Indinavir (Crixivan) -- Nelfinavir (Viracept) - Fusion Inhibitors -- Fuzen - binds to gp41 preventing the binding of HIV. |
|
|
What is HAART Combination Therapy?
|
- Prevents resistant strains development
- Use of HAART (Highly Active Anti-Retroviral Therapy) |
|
|
What is Selzentry?
|
(Marvaviroc) CCR5 Inhibitor stops the R5 virus on the outside of the cell.
|
|
|
HAART therapy can cause?
|
Peri-Anal Herpes II.
|
|
|
AIDS can cause the following infections to come up:
|
- Acute Retroviral
- Kaposi's Sarcoma - Disseminated Cryptococcus - CMV Retinitis - Pnemocystis Carinni Pneumonia (PNP) - HSV infections, male and female genitalia (reactivation and dissemination during HIV infections). - Papillomavirus: Genital Warts See lecture 37-38 for details on these diseases. |
|
|
How can you diagnose Pneumocystis Carinii Pneumonia ?
|
Gromori-Grocott Stain and I.F.A. can be used to diagnosis.
|
|
|
What are the CNS infections statistics?
|
- Acute Infections = high morbidity / mortality
- Fatality ~ 25% in adults - ~10% survivors are left with persistent neurological defect(s). |
|
|
Infections which affect the covering of the brain is called?
Infection affecting the parenchyma is called? Infection affecting the spinal cord is called? If all three areas are affected it is called? |
- Meningitis
- Encephalitis - Myelitis - Meningeoencephalomyelitis |
|
|
1. Brain abscess does not occur from?
2. Meningeal inflammation affects vessels in the? 3. What factors can affect/reduce the blood brain barrier? |
1. Viruses
2. Virchow-Robin spaces 3. Hypertension, High Osmolality, Microwaves, Radiation, Trauma, Inflammation, Ischemia |
|
|
What is the overall goal to management in menengitis?
|
- Early Recognition
- Prompt Intervention - Rapid Determination of Etiologic Agent (s) - Anticipate + Manage the complication |
|
|
What is the 3 Stage Decisions Process?
|
- 1st 30 min critical initial clinical assesment
- 1-2 hours obtain sample for CSF analysis - At 24-48 hours review results of CSF culture. |
|
|
What is the pathology of menengitis?
|
- can be via bactercemia / viremia
- Occurs through seeding of meninges if the organism escapes into the CNS tissue. |
|
|
With meningitis in CSF sample you except?
|
- WBC in CSF
- Predominant WBC = mononuclear, polynuclear or both - If acute - onset over period of hours to days - if chronic - symptoms and CSF pleocyosis persist > 4 wks. |
|
|
What is the difference between viral and bacterial meningitis?
|
Viral Meningitis = "Asceptic"
- Usually self - limiting - Occasionally causes severe infection - Most COMMON causative organism - Bacterial Meningitis = " Septic " -- Usually causes brain damage, hearing and learning disability and sometimes death. |
|
|
What are meningitis symptoms?
|
- Signs are symptoms in the ADULT?
+ Headache - 21-81% + Fever - 59-100% + Confusion - 57-92% + Nuchal Rigidity - 57-92% Patients may elicit seizures if the disease progresses. |
|
|
What is the pathogenesis of viral infections in the CNS?
|
- Viruses have several possible pathways to involve in the CNS.
-- Infect intra-axonally thr a neuronal route like rabies -- infect thru olfactory nerve such as in Herpes -- but MOST COMMON entery is hematogenous Cytokine responsible for viral penterance of BBB is the TNF-alpha. |
|
|
What is a neutropic virus?
|
They have both neurovirulence and neuro invasiveness factors.
--> These determine whether a virus cause disease and where it attacks ------> External Viral Proteins often determine tissue tropism --- Clinical Neurologic Syndrome -------> Relate to specific brain regions we well as the brain cell populations affected by the virus. |
|
|
What was the old common cause of meningitis, and what is the current common cause?
|
- H. Influenza was the old (pre 1990s)
- Strep. Pneumoniae and Neisseria meningiditis leading casue of bacterial meningitis. |
|
|
What are classical meningitis signs?
|
- Stiff Neck
- Kering's Sign - limitation of straightening of the leg with the hip flexed. - Brudzinski's Sign - involuntary flexion of hip and knee - Fever - Headache |
|
|
What is encephalitis?
|
- A neurologic syndrome defined as inflammation of the brain paranchyma
Etiology: Viral Causes, Bacteria is RARE [exception are legionell pneum. borrelia burg, and teponema pall] - Yeast - Cryptococcus Neof. - Parasites - Plasmoid Falciparum and Tryponosomas. |
|
|
What are the symptoms of encepholitis?
|
Acute headache, fever and disturbed leel of consciousness. May have seizures and focal deficits.
NO NUCHAL RIGIDITY Note: Encephalitis is considered, clinically, worse then viral maningitis. |
|
|
Abscess and Empyema, what is it?
|
Usually a result of bacterial infection, and are usually diagnosed by location. Pressure from the exudates accumulaton can damage the CNS tissue possibly permanently. FATAL if not treated.
Basic Difference: Abscess: --> Fixed Boundaries Empyema: --> Lack definable shape and size e.g. spinal epidural abscess is located above the dura mater and a cranial subdural empymea is located between the dura mater and the arachnoid. |
|
|
Describe How Abscess and Empyema are formed:
|
- Direct Extension
- Metastasis with hemategenous Spread - Trauma - Surgical - Immunocopramised Host - Male:Female ratio is 2:1 - 90% of lesions resolve with medical treatment alone - Therapy lasts for 6-8 weeks - "Ring" lesion may persist weeks past ATB therapy. |
|
|
Describe the Pathogenesis of Abscess:
|
Early-Interm Cerebritis:
--> Early infection + inflammation, poorly demarcated, perivascular infiltrates Late Cerebritis: --> Reticular Matrix (collagen Precursor) + Developing Necrotic Center Early Capsule: Neovascularity, Necrotic Center Surrounded by reticular network Late Capsule: Collegen Capsule, Necrotic Center, Glosis around the Capsule = "POP" |
|
|
What is the most common organism in abscess formation?
|
- Streptococcus most common, 30-50% (aerobic or anaerobic).
- 80-90% multiple organisms - Infants - gram negative organisms - Other causative agents: Fungi and Parasites |
|
|
What is the etiology/cause of Empyema?
|
- 45-50% contiguous suppurative (sinus)
- 25% hematogenous from a distant source - 10% trauma |
|
|
What are the symptoms of brain abscess or empyema?
|
Caused by a combination of:
- Increased intracranial pressure (headache) - Infection (fever) - Focal Neuralgic Brain Tissue Damage. |
|
|
Describe Fungal Brain Abscess?
|
- Disseminate hematogenously from a remote infection site
- Create multiple areas of infection within the brain and other organs - Meningeoencephalitis occurs early by vascular invasion by the fungus - Can see secondary thrombosis, cerebral infarction and hemorrhage. Fungi: - Aspegillus - Candida Albicans - Cryptococcus Moncytogens Diagnosis: Aspergillus - branching hypha: classical apperance Candida - forms granulomatous reaction with central suppuration. Yeast forms my be seen with silver stain (Surgery best mode of treatment) Cryptococcus - fungi appear like encapsulated spheres. Capsules NOT seen on H+E, but stain with PAS and mucicarmine. |
|
|
Quickly Describe the Parasitic Brain INfections:
|
Taxoplasma Gondii:
-- Common in immunocompramised -- Are found free in tissue or inside the inflammatory cells, neurons, etc. -- Can produce a meningeoencephalitis, abscess, hemorrhage or granuloma. Diagnostics: - Must demonstrate toxoplasmosis in tissue or be serologic study Etamoeba Hysolytica: - RAPIDLY cause abscess - Very little capsule formation |
|
|
Describe the CSF values for Bacterial, Viral and Fungal Infection:
|
Bacterial Infection:
Elevated Opening Pressure WBCs: >1000 Protein: Mild to Elevated Cell Differential: PMN Viral Infection Opening Pressure is normal, WBCs <100 Protein: Normal to elevated Cell Differential: Lymphocytosis Fungal INfection: Opening Pressure is Variable WBC's Variable Protein: elevated Cell Differentiation: Lymphocytosis |
|
|
What is the most common pathway of enterence for viruses into CNS?
|
- Hematogenous (bloodborn)
- Infection through olfactory nerve through HSV - Infection intra-axonally through neuronal route such as rabies |
|
|
What is the CNS lab result of viral manangitis?
|
- Protein is moderatly high
- Glucose is normal - White Cell Count = increased but not as much as bacterial White Cells are mostly lymphocytes. NOT NEUTROPHILS NO GRAM Stain |
|
|
What are the common viral causes of meningitis?
|
- Enteroviruses (particularly ECHO)
- Coxsackie - Poliomyelitis virus - Can be a complication of mumps, herpes simplex virus. |
|
|
What is an Enterovirus?
|
Piccornaviridae Virus (ssRNA)
|
|
|
What are polio and non-polio viruses?
|
2 distinct classes of enteroviruses:
non polio-viruses: - Coxsackie, enteroviruse and unclassified enteroviruses. polioviruses: - types 1,2 and 3 Pathogenesis: - Antibody production to enteroviruses occurs first 7-10 days - Increases during the recovery within 2-4 weeks. - The infection can progress to CNS involvement EITHER during major viremic phase or at a later time. - GI cells - viral receptors and seeds into the bloodstrem by day 3. Can replicate in liver, heart, skin, meninges, lungs and adrenal glands |
|
|
Define the epidimiology of Enterovirsues?
|
- account for 80-85% of viral pathogens identified via PCR
- Fecal-Oral spread during the warm summer month - Water sources during summer-fall - Infants and children MOST susceptible - Usually self-limiting and no specific antiviral therapy - there are over 10,000 cases reported each year - MOST COMMON causative agents of viral meningitis. |
|
|
What are Enterovirus associated clinical syndromes:
|
- Commonly encountered infection especially in infants and children
---> Hand foot and mouth disease (HFM) --> Herpangina* --> Myocarditis* --> Pleurodynia* * Caused by Coxackie B virus * |
|
|
What is Enterovirus CND Infection:
|
- 60% asymptomatic or subclinical
- Patient symptoms can be seen as benign as an uncomplicated cold or as threatening as an eseptic CNS infection Usually MENINGITIS occasionally encephalitis with permanent damage. |
|
|
Describe the Coxsackie Virus:
- What does GROUP A cause and GROUP B cause? in terms of Paralysis |
-Non-Enveloped ss linear RNA
- Worldwide - Oral-fecal, respiratory + fomites - Group A: -- Causes flaccid Paralysis -- Skin and Mucous membrane = herpangina, HFM Group b - Cause SPASTIC paralysis - Cause Pleurodynia, myo/pericarditis, hepatitis Both Group A and B: - Cause non-specific fever, rash, URI, aseptic meningitis. |
|
|
What is Coxsackie Aseptic Meningitis:
|
> 90% of viral aseptic meningits is caused by:
- Coxsackieirus B (Serotype 2-5) - Echoviruses Onset is rapid OR gradual - Fever, chills, n/v, neck pain, photophobia, rash, upper respiratory symptoms Complications in 5-10% including encephalitis: - Lethargy, Seizures, Coma, movment disorders. - Infants < 1 y.o have highest incidence of aseptic meningitis - No long terms deficits appear to exist with aseptic meningitis |
|
|
Describe Coxsackie Encephalitis:
|
-An unusual manifestation in association with aseptic meningitis:
- These patients present with cerebral dysfunction: -- Altered mental status, personality changes and neurologica deficits -- Motor, Sensory, Speech Impairments -- There is often a history of RASH -- Symptoms may be preceeded by a history of upper respiratory infection within the past 7-14 days. |
|
|
What clinical conditions are associated with Coxsackie Virus?
|
- Hand foot and mouth disease
- Herpangina - Epidemic Pelurodynia - Acute Hemorrhagic Conjunctivitis |
|
|
What is the Diagnosis for enteroviruses?
|
- CSF specimen
- PCR + for enterovirus (helps avoid treting for bacterial infection) - PCR + in non-CNS infection site lesions, throat, stool speciment/biopsies - EEG (for encepholitis) |
|
|
What is a Picornaviridae Polio Virus
|
- Unapparent infections
- 95% asymptomatic, virus in RES - diagnosis - isolated from feces or oropharnx with + serum antibody - Abortive polio "minor illness" - Similar to any systemic viral illness - Nonparalytic Polio -- Similar to other enteroviral causes of meningitis - Paralytic Polio (<1% of infections) -- Spinal - flaccid paralysis (lysis of anterior horn cells) --- Bulbar paralysis of Cranial Nerves IX + X, medullary/respiratory centers - Polioencephalitis - RARE |
|
|
What is PPS - Post Polio Syndrome:
|
- Infection people develop several decades after a polio virus.
- PPS is not necessery re-infection, it can be that old nerves that were reconnected after the orignal infection break down / become weaker, so you have muscle weakness. |
|
|
What is the treatment for polio?
|
Pantacel is an active immunization for: diphtheria, tetanus, pertussis, poliomyeltis and Haemophilus Influenza.
|
|
|
What is the Lymphocytic Choriomeningitis Virus (LCMV)
|
Arenaviridae:
- Uncommon - Usually spread through contact with excreta (like pet hamster) - No human-human transmission transplants are ok - Noncytopathic virus, - immnopathological CD-8 T Cells |
|
|
Give a brief description of the herpes viruses?
|
- HSV 1 and 2, VZV, CMV, EBV, HHV 6,7 and 8.
- Account for less then 3 % of all meningitis cases - Estimated 2-4 million cases per year - HSV-2 is the MOST common and is associated with primary genital infections - VZV infection may occur with or without typical skin lesions. |
|
|
What is Herpes Simplex Encephalitis?
|
- Most common and sporadic cause of encephalitis
- Primarily due to HSV-1 (Adults) - Babies, generally fatal, HSV II - Causes destructive focal encephalitis in anterior lobe and orbital frontal region. Symptoms: - Early personality changes, bizzare behavior, hallucinaions, followed by seizures and hemiparesis. -- 2-4 cases per million per year ---> 1/3 of herpes encephalitis are due to primary HSV ---> 2/3 are due to re-infection or re-activation of a latent HSV agent --> Most fatal cause in chidre |
|
|
How do you Diagnose Herpes Simplex?
|
Spinal Fluid:
- RBCs as well as WBCs (pinked tingled spinal fluid) - HSV + PCR MRI, CT Scan, Localizes are inflammation: - Shows distinctive pattern Clinical Symptoms-trigeminal N.root ganglion with extension of temporal loe-classic EEG changes = one lob This IS a treatable Disease: - Acyclovir IV over 10 days -- Inactivates viral DNA synthesis -- May have 10% replace following completion of treatment -- Famcyclovir is an alternative -- Rebetrol being trialed in children |
|
|
Describe Herpesvirus Vircella (VZV) Chicken Pox?
|
- DNA virus
- NOT a true pox Virus - The infected person is the sole reservoir - Must ISOLTE the patient -- organism is NOT resistant to drying and can survive outisde the body like the true pox virus CNS involvement can accompany or follow varicella as a RARE complication. A FEMALE who gets infected during PREGNANCY can have a newborn with birth defects, microcephaly, motor disability, cataracts, etc. |
|
|
When does VZV cause encephalitis?
|
in AIDS, histologically it looks like HSV encephalitis. Causes fused necrosis in cerebral cortex.
|
|
|
Give a brief description of the herpes viruses?
|
- HSV 1 and 2, VZV, CMV, EBV, HHV 6,7 and 8.
- Account for less then 3 % of all meningitis cases - Estimated 2-4 million cases per year - HSV-2 is the MOST common and is associated with primary genital infections - VZV infection may occur with or without typical skin lesions. |
|
|
What is Herpes Simplex Encephalitis?
|
- Most common and sporadic cause of encephalitis
- Primarily due to HSV-1 (Adults) - Babies, generally fatal, HSV II - Causes destructive focal encephalitis in anterior lobe and orbital frontal region. Symptoms: - Early personality changes, bizzare behavior, hallucinaions, followed by seizures and hemiparesis. -- 2-4 cases per million per year ---> 1/3 of herpes encephalitis are due to primary HSV ---> 2/3 are due to re-infection or re-activation of a latent HSV agent --> Most fatal cause in chidre |
|
|
How do you Diagnose Herpes Simplex?
|
Spinal Fluid:
- RBCs as well as WBCs (pinked tingled spinal fluid) - HSV + PCR MRI, CT Scan, Localizes are inflammation: - Shows distinctive pattern Clinical Symptoms-trigeminal N.root ganglion with extension of temporal loe-classic EEG changes = one lob This IS a treatable Disease: - Acyclovir IV over 10 days -- Inactivates viral DNA synthesis -- May have 10% replace following completion of treatment -- Famcyclovir is an alternative -- Rebetrol being trialed in children |
|
|
Describe Herpesvirus Vircella (VZV) Chicken Pox?
|
- DNA virus
- NOT a true pox Virus - The infected person is the sole reservoir - Must ISOLTE the patient -- organism is NOT resistant to drying and can survive outisde the body like the true pox virus CNS involvement can accompany or follow varicella as a RARE complication. A FEMALE who gets infected during PREGNANCY can have a newborn with birth defects, microcephaly, motor disability, cataracts, etc. |
|
|
When does VZV cause encephalitis?
|
in AIDS, histologically it looks like HSV encephalitis. Causes fused necrosis in cerebral cortex.
|
|
|
Describe Cytomegalovirus CMV - HHV5
|
- spread via rep. droplets, blood, urine, feces, mucus.
- 50% of young adults are infected - Poses no real threat to immune competent - Dorment - Inants infected in utero - hearing and brain damage - no risk if infected after birth - High Risk population are: HIV, AIDS, organ transplant patients. |
|
|
What are the symptoms of CMV - HHV 5? Diagnosis?
|
Mild flu-like conundrum (actually infect any organ)
- Lethargy, Fever and drop in WBCs - Encephalitis - Convulsions and coma are associated. Diagnosis: - Blood, Sputum and Urine Tests (as the virus shed in all these) - Mild drop in WBCs - Neuron - show intranuclear inclusions (HE called "OWL's EYES"). - Mainly involves periventricular parenchyma - Retinitis is often associated Treatment: - Depends on severity of condition - Fluids, bed rest - Gancyclovir, especially with CNS infection or transplant history. |
|
|
What is Epstein-Barr virus EBV?
|
- Herpes Virus
- Worldwide - 95% of adults between 35-40 are infected - Infants become susceptible as soon as maternal antibody disappears - 35% - 50% EBV infection in adolescence cause infectious mononucleosis . Symptoms: - CNS symptoms are rare, HOWEVER, 7% of hospitalized patients with EBV infection, had significant neurologic complications including aseptic meningitis and encephalitis. - 50% only present with a headache. |
|
|
What is the HHV-6 Human Herpes Virus?
|
- Known as Roseola in children (rash)
- Can be life-threatening if reactivated in immunocompramised - Infects T Cells and NK cells Has shown to be associated with chronic disease including MS and Chronic Fatigue syndrome. Can cause serious CNS infection in bone marrow transplant patients. |
|
|
What is the monkey pox semian B virus?
|
- Herpes Virus Simiae
- found in semian species but they have no symptoms - RARE but FATAL - 80% die due to complications - Survivors often have serious neurological sequele - RISK POPULATION: Vets, Lab Works and people who have contact with monkeys. Treatment: Acyclovir (maybe) - no clinical evidence |
|
|
Describe Paramyoviridiae [ MUMPS ]
|
- Accounts for 10-30% of non-immunized patients develop CNS infections.
-- Meningitis and encephalitis are both seen - Occrurs in Winter and Spring - 40-50% no clinical parotitis Typical Mumps Symptoms: - Fever, swollen parotid glands - Malaise - NO rash or pox - 10% meningitis complications |
|
|
Describe HTLV-1 CNS role?
|
- Slowly progressive SPASTIC paralysis
- Lesions located in white matter w/ concomited demyelination |
|
|
What is Measles Encephalitis?
|
- Typically Self-Limiting
- Associated with: - SSPE (Subacute Sclerosing Panencephalitis) ++ causes BEHAVIOR CHANGE in kids (watch out in school) - Boys:Girls 3:1 - 10% Fulminant course, 10% chronic course - in SSPE death occurs in 1-3 years. Acute course: 3 month Chronic corse 4-10 years. |
|
|
Describe Rubella?
|
- Progressive Rubella Panencephalitis (PRP)
- VERY RARE and slow - males age 8-19 - Can recover from brain and leukocytes of infected patients. Symptoms: -Beh. problems in school -ataxia, dementia spastic quadriparesis -clinical course more protracted then SSPE - KOPLIKs SPOTS - Lymphadenopathy - Maculopapular Rash |
|
|
Describe Rabies: Rhabdoviridae
|
- Transmitted: scratches, abrasions, open wounds, mucus membrane of contaminated salaiva
- urine, feces, blodo of infected person is NOT prophylaxis - Domestic animals count for <10% of rabies cases - Majority of reported cases are from wild animals |
|
|
Describe Rabies: Lyssavirus
Describe the Symptoms and Diagnosis |
- 'bullet' shaped virus
- 5 proteins N,P,M,G and L - Helical ribonucleoproteins (RNP) and envelope - Attaches to the cell via absorbtion - Virions, preference for nerve and salivary gland cells. Symptoms: Early: General Malaise, Fever and headache Progressive: neurologic symptoms to include: insomnia, confusion, slight or partial paralysis, agitation, hypersalivation hydrophobia and dysphagia. - Death occurs within days of symptom onset Diagnosis: - Saliva - Virus isolation by RT-PCR - Serum and CSF for rabies virus antibodies - Skin biopsy for rabies antigen in cutaneous nerves at base of hair follicales - Brain tissue of animal histopathology: -- Negri Bodies -- Lymphocytic Foci: babes nodules consisting of glial cells -- Mononuclear infiltration: perivascular cuffing of lymphocytes or PMN's. Treatment: - Prevention - Wash all wounds with soap and water - PEP = 1 dose of immune globulin and 5 doses rabies vaccine over 28 day period. |
|
|
Describe Recurrent Benign Lymphocytic Meningitis:
|
- Mollarets Meningitis
- Multiple episodes (3-10) lasting 2-5 days - Spontanious recovery, 50% have transient neurologic symptoms - 95% cases due to HSV-2, 5% due to HSV-1 |
|
|
Describe Retroviridae HIV-1
|
- clincaly silent
- 5-10% occurs w/ primary infection (Acute HIV syndrome) - May be persistent or recurrent Can lead to encephlapathy: AID/HIV dimentia. |
|
|
What CNS symptoms do you get with HIV?
|
Acute - HIV infects the meninges and cause meningites
Once HIV progresses to AIDS status causes sub-acute encephalitis: -- Symptoms: resemble pre-senile dementia -- Brain shrinks, ventricles enlarge -- Differential: multifocal leukoencephalopathy caused by JC virus Early treatment with ARVs may circumvent this sequelae and even be CNS protective long term. Encephalitis + HIV = Multinucleated Giant Cells. |
|
|
What are the symptoms of Polyoma Virus JC Virus - PML?
|
- Progressive Multifocal Leukoencephalopathy
- Causes subclinicl infection -- Its DNA is in the healthy human tissue -- Majority of humans worldwide have been infected -- Can cause symptomatic lymphoma Progressive Multifocal Signs: hemiparesis, visual loss, aphasia, seizures, dementia, personality changes and gain problems Death is invariable and occurs within weeks to months of clinical onset. Neuroimaging shows a very characteristic white matter lesions commonly posterior to the perietal occipital area. |
|
|
What pathogens are responsible for community aquired meningitis?
|
- H. Influenzae, S. Pneumoniae, N. Meningitidis.
--> Estimated 1.2 million cases annually with 135,000 deaths --> Sub-Saharan Africa, 1996-1998 300,000 cases with 30,000 deaths due to meningococcal disease Highest attack ates are in youn and previously well. |
|
|
Describe the Arthropod-Borne Viral Encephalitis
|
They can be:
- Eastern Equine Encephalitis, VEEm JBE, nd LaCrosse St. Louis - Transmitted by ticks - Cause sporadic and epidemic encephalitis - Complication in children primarily seizures - Worldwirde |
|
|
What are the Togaviridae - Eastern Equine Encephalitis?
|
- Culiseta or Aedes mosquito bite.
- Uncommin in humans, but Aedes may act as bridge - High mortality - North America Pathophys: - Diffuse CNS involvment - lg. # of active viruse enter the brain paranchyma and perivascular areas mediating damage, these include: -- infiltrating neutrophils and macrophages, focal necrosis and spotty demyelination. -- vascular inflammation w/ endothelial proliferation, small vessel thrombosis and perivascular cupping may also develop. - EEE affects: perikaryon and dendrites of neurons w/ minimal glial cell infiltrate. Many patients who die late in the disease exhibit diffuse cerebral atrophy particularly the cortex. Symptoms: - Prodrome - fever, chills, weakneess, headache, myalgia, --> due to viral replication in non-neuronal tissue --> Rapid Progression: HEADACHE (most prevelant), nuchal rigidity, confusion, somnolence, seizures and coma. ---> due to cell spread thru microvascular permeability of brain, then cell-to-cell occurs via axons and dendrites. |
|
|
Describe the Western Equine Encephalitis [WEE]:
|
- Culex mosquito bite but Aedes can also carry
- Genus - US in summer time - Neuroltropic alphavirus which causes encephalitis and viral syndrome WITHOUT rash Fatality rate is 3-4% with children haveing a 30% chance of CND sequelae. WEE Symptoms: - Initial symptoms fever, headache, chills, (viral prodrome) 1-4 daus and somnolence, coma and death in 1-2 days. - Diffuse CNS involvement - Damage mediated by large number of immunologically active cells that enter the brain - Neutrophils and macrophages infiltrate the brain parenchyma causing neuronal destruction, focal necrosis and spotty demylenation - Vacular inflammation with small vessel thrombosis may occur - Cell death by apoptosis primarily in the glical and inflammatory cells |
|
|
Descrbe Venezuelan Equie Encephalitis - VEE:
|
- Culex and Aedes mosquito bite.
- Potential BIOLOGIC weapon - Clinical symtoms similar to the other "EE" - no death, only immunit ydue to immune response in humans, in horse mortality is 80%. |
|
|
Describe the Flavividridae St. Louis Virus (SLEV)
|
- Culex Mosquito
- Probability of CNS infection depends on: --- Efficency of viral replication at extraneural sites --- Degree of viremia --- Age of the host Pathophysiology: - Virus enter throught cerebral capillary endothelial cell/astrocyte complex (BBB) - Or across fenestrated endothelium in areas that CNS does not have the usual BBB capacity (i.e. Choroid Plexus) Symptoms: - Mortality - 2-20% - Prodrome- Malaise, Fever - 20% develop sequelae: , irritability, memory loss, movement disorders, motor deficit.s. - Seizures and coma are COMMON - Defervescence over several days, and rare retrograde tracking from a peripheral site - NO chronic clinical illness or replase but 25% develop cranial nerve palsies. |
|
|
Describe Japanese Encephalitis Virus (JEV)
|
- Flavirus
- Culex Masquito - Incubation 4-14 days - Rural areaso of Asia - Traveler's infections risk estimated at 1 case per 150,000 person-month - Worldwide ~ 35,000 - 50,000 per year Symptoms: - Viral Prodorme - Fever by 2nd week - Encephalitis Syndrome with tremors NOT seizures - Most symptoms defervesce, but ~50% relapse 1 year later - Low IgG/IgM ratio (CSF) = death risk |
|
|
Describe West Nile Virus?
|
- Flaviviridae
- Life cycle = wild birds (reservoir), mosquito (vector) - Only a RARE a few person-person trasmission has been confirmed - 3-15% fatal - REPORTABLE disease SYMPTOMS: - Viral Prodrome with maculopapular rash of trunk and extermities - Severe Infections - headache, HIGH fever, nuchal rigidity, stupor, LOC changes, tremors, seizures paralysis. - Rare deaths (young and old) Diagnosis: - Clinical Findings: -- HIGH fever (>38C), LOC changes, other CNS signs. -- CSF: -- Negative bacterial strain -- Pleocytosis (WBC b/w 5-1500 cells) -- Elevated Protein (> 40 mg) |
|
|
Describe La Crosse Virus?
|
* Bybtavurudae *
- Forested areas are more prone in upper Midwestern states - Severe disease occurs more commonly in children and characterized by seizures, coma, paralysis, and other neurologic sequelae. - Death occurs in < 1% cases. Usually spontanseously resolves. |
|
|
How does the bacterial invade the CNS?
|
- Invasion from nearby infection
-- Middle ear or chronic sinus infections - Spread from distant sites -- Hematogenous - RARE - trauma or surgical procdedures or unrecognized penetrations --- Direct introduction into the CNS. Note: sometimes the source can't be identified. |
|
|
Define the pathogens in bacterial Meningitis:
|
- Neonates: Group B strep, coliforms, and listeria monocytogens
- Infants: N. meningitis, Strep. Pneum. H. Influenza, - Children: Strep. Pneum and Neisseria meningiditis - Strep. Pneumoniae - ALL AGES - >75% caused by Neiss. Mening., Strep. Pneum., and H. Influenza |
|
|
What are the causitive Agents of Bacterial Meningitis:
|
- H, Influenza, Serotype B (not much problem since polio vaccine)
- Strep. Pneumoniae - Neisseria Monocytogenes - Strep Agalactiae - Aerobic Gram - Bacilli - Staphylococci (S. Aureus, S. Epidermidis) neg. coagulase - Staphylococci - Mycobacteria Tuberculosis - Spirochetes - Brucella Abortus |
|
|
Describe Bacterial Virulance Factors:
** KNOW THIS ** |
- N. Meningitis
---- Capsule, IgA protease, pili, endotoxin. - H. Influenzae ---- Capsule, IgA protease, pili, endotoxin - Strep. Pneumoniae ---- Capsule and IgA protease only |
|
|
What are the symptoms of bacterial maningitis by age?
|
- Babies:
-- No Obvious Clinical Signs -- Failure to Thrive -- Fever vs. Hypothermia -- Occasionally Diarrhea - Adults and Children: -- Fever, Emisis, LOC change, new rash that looks like a bruise, recent URI (e.g. Sore Throat), Brudzinksi's sign |
|
|
What are the generap Prevention Options for bacterial maningitis?
|
Vaccines:
- HiB - N. meningidits (4 strains) not mandatory - Many types of Strep. Pneumoniae Recommendations for Vaccines: - College Freshman and Oversease travel and infants - Infection are reportable to Health Deparments. |
|
|
Describe N. Meningitidits:
|
- Exclusivley HUMAN pathogen
- Gram Negative seen intracellulary in PMN WBC's (skin lesions and CSF smear) - Waterhouse Fredrickson Syndrome Epidimology: - High morbidity and mortality - 1.2 million cases / year - 50% survirvors have neurologic or other sequelae - 30% carried in healthy populations - Overcrowding increases carriage rate ---- Military, Boarding Schools, Colleges - Spread via infected droplets - Acute Infection = Highest Risk ~ 24 ours. |
|
|
What can increase the rate of transmission of N. Meningitis?
|
Smoking
|
|
|
Describe Meningococcal Meningitis?
|
Characteristic rash of meningococcal septicemia.
Diagnostics: - Clinical Signs: especially rash, sepsis, fever with typical meningitis symptoms - CSF tap is + for organism - Tumbler Test = special glass, put against the rash, if you see the rush, it means menigiococcal meningitis. Options for Treatment / Prevention: - Penicillin (DOC) or 3rd generation cephalosporin (these may be combined with cloramphenicol) - Tetravalen Vaccine for Groups A, C, Y and W - Vaccines for N. Mening, Hib and many types of Strep. Pneum. Availbable. |
|
|
Describe H. Influenzae Meningitis:
|
- Window of infection opportunity between 4 months and 3 years old
- Close at-risk contacts should receive prophylaxix treatment Pathogenesis: - Gram negative encapsulated pleomorphic organism - Spread vis blood stream from respiratory tract to the brain. Symptoms: - Insidious - Carries a greater risk of permanent neurologic damage than any other bacterial meningitis - Treatment and Prevention: -- Ampicillin if the isolate does not produce B-lactamase -- 3rd generation cephlasporin and chloramphenicol are alternatives -- Vaccine made with the type B capsular polysaccharide ----> Only 21 cases reported in US in 2007. |
|
|
Describe Listeria Monocytogenes:
|
- Gram +ve non-spore forming aerobic rods
- Can be Zoonotic - Food-born outbreaks (dairy and deli) although in the human there can be a resevoir for the organism in the intestine - 2-12% of human carry this organism - Can be transmitted mother to child during delivery (and can cause spontaneous abortion). Pathogenesis: - Grows in macrophages ( great for spreading around the body). - "Internalin" - cell attachment molecule - "Listeriolysin" - protein that heps is move within the cell - Uses cellular actin to move to new cells - Likes to infect Immunocompromised. Diagnosis: - Intracellular gram +ve rods in macrophages and neutrophils - CSF culture = look like beta- strep, but are catalase +ve and "tumble" Treatment: - Ampicillin combined with gentamycin - Penicillin G, erythromycin and chloramphenicol are also useful |
|
|
Describe Streptococcus Agalactia?
(GBS- group B Strep) |
- Normal female genital organism
- 60% of babies die who develop GBS dring the first week of life - In late 20th century was leading cause of death and disease in newborns - W/ out precautions, 1-3 cases occur per 1000 births Symtptoms specific GBS: Neonate: - Lethargy, Fever, Spesis, and respiratory distress Children and Adults - Puperpeal fever, and other skin and soft tissue infections. Diagnosis of GBS meningitis: - Gram +ve coci or cocobacilli in chains on culture - Can use speciic antibody or a DNA probe for group B -- Most accurate way is to test for the Lancefield Group B antigen on the surface of bacteria. Treatment and/or Prevention for GBS: - Penicillin for mother if culture positive - IV penicillin for infant, especially if lethargy is noted! |
|
|
Describe Strep. Pneumoniae:
|
Occurs at any age but more likely in:
-- Children < 2 years old -- Elderly High Mortality -- 20-30% with treatment -- 15-20% survivors with permanent neurologcal damage |
|
|
Describe Strep. Pneumonia meninigitis:
|
- Major most common cause of bacterial meningitis
--- Highest cause of neonatal meningits - May follow pneumonia especially in elderly - Some strains are becoming resistant due to mutations in their PBP (Penicillin Binding Protein). Symptoms: - Typically Meningitis Symptoms - Clinical History May Include: --- May follow pneumococcal Pneumonia --- Middle Ear Infections --- Sinusitis Treatment and Prevention: - Vaccine - recommended for >65 y.o and those < 2 y.o (also essential in those w/ Sicke Cell Disease) - Antimicrobials -- High dose penicillin may work -- 3rd generation cephalosporins or vancomycin required if ANY resistance of peniclling resistance. Enterobacteriacae - Neonates at highest risk - "Birth Canal Meningitis" Common Organisms: - E. Coli, Especially strain K1 -- 28,5% of all neonatal cases - Klebsiella Enterobacter Epidimology: - 0.3 per 1000 live births - 41.5% have no residual problems after recovery - 8% mortality rate - May travel from nasopharnynx hematogenously to the meninges - During pregnancy, increased colonization of K-1 strain, terefore they are a high cause of neonatal meningitis Symptoms: - < 1 month old - irritability, lethargy, vomitin, lack of appetite and seizures. - 4-18 months - neck rigidity, tense frontanels and fever - Older children and adult - headache, vomiting, confusion, lethargy, seizures and fever Diagnosis: - K-1 capsular antigen - Gram negative bacilli single or pairs - Facultatively anaerobic with both fermentative and respiratory type of metabolism Treatment: - Beta-Lactam antibodies: -- Ampicillin -- Cephalosporin |
|
|
Describe Klebsiella Enterobacter?
|
** Poverty Associated Meningitis **
- May be early onset < 3 days - 2nd only to GBS - May be late onset 8-28 days - 2nd only to Staphylococcus - Higher incidence noted in cockroach infected areas Symptoms: - Not doing well, lethargy, poor feeding, little cry, hypothermia, fever, sclerema (induration of adipose tissue). Diagnsis: -Culture of blood, CSF, urine and local infections -C-reactive protein - positive - WBCs: -- Thrombocytopenia -- Early - Leukopenia and neutropenia -- Late onset - Leukocytosis and neutrophilia Treatment: - After Cultures: -- Start antibiotics with ampicilln and gentamicin (or Amikacin, Tombramycin, Netilmicin) -- Third Generation cephalosporins (Cefatoxamine, Ceftazidime, Ceftraxone) may replace gentamicin. |
|
|
Describe Rickettsia Ricketssi (Rocky Mountain Spotted Fever)
|
- Tick Bite - NOT spread by person to person
Diagnosis: Clinical Picture: - Key = rash - Headache - some CNS infections Treatment: - Prevention - Remove the tick within 6-12 hours - Generic treatment for rash, and systemic inflammatory symptoms - Killed vaccines only for military and researchers (not public). |
|
|
Describe Erlchia Chaffeensis:
|
- 480 cases in US in 2006
- Occasionaly FATAL - RASH is NOT common Symptoms: - Mild to Severe multisystemic symptoms - Fever - Hypotension - Cofusion due to CNS infection - Renal Failure - Heptaocellular Injury Diagnosis and Treatment: - Antibody testing of acute and convalesent sera -- Shows increase antibody titer to organism - Grows in macrophages causing monocytic erlichiosis - PCR Treatment: - Tetracyclin is a drug of choice |
|
|
Describe Coxiella Burnetti - Q Feer:
|
- Zoonotic and Distrubuted Globally
- Reportable Disease in US - Most infections are asymptomatic - Resistant to heat drying and many disinfectant - Inhalation of contaminated dust Symptoms: - HIGH fever (38-39 C), lasting up to 2 weeks - Malaise, headache confusion, chills, n/v/d, abdominal and chest pain. Diagnosis: - Serum: -- Thrombocytopenia -- Presence of antibodies -- Indirect Immunoflourescence assay (IFA) -- Also: Immunohistochemical staining of infected tissues - Doxycycline - drug of choice Pathogenesis: - Common in areas of middle cerebral artey. - Commin @ gray-white junctions - Usually not killed by disinfectants |
|
|
Brain absesses occur most frequently because of?
|
- Following Surgical Trauma
- Can also occur due to extending osteomylitis or ear infection or hematogenious spread. |
|
|
in Community aquired meningitis the most common route of transmission of bacterial pathogens to the meninges is:
|
- Pneumococcal Meningitis
- Neisseria Meningitidis Meningitis - Brain Abscess - HSV-1 encephalitis - Rabies |
|
|
In Community aquired bacterial meningitis the most common route of entry to the central nervous system is via the:
|
- Hematogenous route
- Cribiform Plate - Middle Ear - Gastointestinal Tract - Sinuses |
|
|
Until the past decade, H.Influenzae was the leading cause of meningitis in young children (2 month to 5 years) but its incidence has declined because of:
|
- Earlier antibiotic treatment of otis media
- Hepatitis B vaccine - H. Influenza vaccine - Because now the leading cause is N. Meningiditis. |
|
|
Briefly describe the fungal brain infections?
|
- Disseminate hematogenously from a remote site of infection (lung or oropharynx)
- Create multple areas of infection within brain and other organs - Can cause abscesses - Meningoencephalitis occurs early by vascular invasion by fungus - Can see secondary thrombosis, cerebral infarction and hemorrhage Common Fungal Organisms: - Aspergillus - Candida Albicans - Cryptococcus Monocytogenes |
|
|
Describe Fungal Infections and Diagnostic:
- Aspergillus - Candida - Cryptococcus |
- Aspergillus - branching hyphae: classical apperance
- Candida - forms granulomatous reaction with central suppuration. Yeast forms may be seen with silver stains (Surgery best mode of Treatment) - Cryptococcus - fungi appear like encapsulated spheres. Capsules NOT seen on H+E, but stain with PAS and mucicarmine. |
|
|
Describe Cryptococcus Neoformans:
|
- world wide in the soil
- 0.4 - 1.3 cases per 100,000 in US - 2-7 cases annualy among AIDS patients - 85% among HIV with meningoencephalitis - Immunocompromised at highest risk - Sequela of menigitis may lead to permanent neurlogic damage - Mortality rate 12% Symptoms: - Initial Pulmonary Infection is assymptomatic (organism is inhaled) - General Meningitis Symptoms -- Headache -- Fever -- Confusion -- Nuchal Rigidity CSF and Blood cultures: - Direct observation using India Ink smear shows encapsulated yeasts (takes days and may lack sensitivity) - Detection of cryptococcal polysaccharide antigens using immunologic methods (faster, more sensitive and specific). Treatment: - Amphotericin B - And 5 Flucytosine |
|
|
Describe Histoplasmoisis Capsulatum:
|
- 80% of people living in areas where H. Capsulatum is common, test positive.
- Infants, Young Children and older persons in particular those with chronic lung disease are at increased risk for severe disease. - Disseminated disease is more frequently seen in people with Cancer, AIDS or other forms of immunosupression. Etiology: H. Capsulatume grows in soil and material contaminated with bar or bird droppings. Spores become airborn when contaminated soil is distributed. - Breathing the spores causes infection - The disease is not transmitted from an infected person person to someone else. - More common in men - 7-23% mortality rate with CNS symptoms Symptoms: - Occur within 3-17 days (10 average) - Acute respiratory syndrome -- Fever, cough, productive sputum, erythema multiform - Disseminated to deeper tissues if untreated - Acute progressive Syndrome -- 5-20% mortality with CNS involvement Chronic: - Constitutional symptoms with 50-60% mouth and gum pain d/t mucosal ulcers Diagnosis: - CSF -- 30-60% cultre + for organism - Serum studies -- Anemia, 70-90% pancytopenia, alk. Phosphatase, 1:32 complement fixing antibodies + organism culture. Chest X Ray: - May be WNL, chronic = 50% with hilar lymphadenopathy and diffuse nodula infiltrates - CT scan: -- Useful in detecting cerbrl histoplasm before doing LP. Treatment: - Initiate medical therapy for all patients with progressive disseminated histoplasmosis and meningitis In severe cases of CNS infection, administer intrathecal or intraventricular injections in addition to intravenous antifungual therapy. |
|
|
Describe Candida Albicans:
Symptoms Diagnosis Treatment |
Candida albicans is the most frequent clinical manifestation of IC-related CNS Candidiasis
- More common in neonates - 64% of them die from the Candidal CNS involvment - 18-52% of patiens dying with invasive candidiasis were found to have scattered brain microabscesses with little or no meningeal involvement Symptoms: - Adults - fever, headache, nuchal rigidity, altered mental status, confusion and disorientation - Common Feature: prolonged interval between onset of symptoms and diagnosis - Neonates presents as part of the syndrome of neonatal invasive candidiasis bulging fontanelle and splitting sutures. Diagnosis: CSF - pleocytosis between 500 to 600 cells/mm3, lymphocytes or polymorphonuclear preponderance, moderatly low glucose (but only ~ 60% of cases) and mild increase in protein concentration (mean 123 mg/dl, range 30-260 mg / dl) Treatment: - Amphotericin B - 5-Fluorocytosine (but cannon be used alone. Must be in conjunction w/ an antifungal medication) - Fluxonaole (if its full blown meningitis) |
|
|
Describe Coccidioides Immitis:
|
- 1300 cases reported in 2006 US
- Confined to the Americas - Endemic in south-western US, Mexico and parts of South America - Causes CHRONIC meningitis - Inhaled of airborne arthrocondia after disturbance of contamined soil by humans or natural diseasters (911, dust storms, earthquakes, etc) - High Risk Persons: -- Construction Workers -- Agricultural Workers, Farmers -- Archeologists Symptoms: - Flu-like symptoms -Failure to recover --- Chronic Pulmonary Infections -- Widespread Disseminated Infections -- Meninges, Soft Tissues, Joints, Etc. -- Meningitis may lead to permanent neurlogic damage. Treatment: - Cocci are treated with: -- Amphotericin B and Miconazole. |
|
|
Describe Aspergillosis Fumigatus:
|
- Found in the soil, decomposing plant matter, ornamental plants food items.
- Transmission - inhalation of airborne conidia (spores) - May be considered nosocomial infection associated with dust exposure during building or rennovation. - Also occasionaly from a biomedical device. Three RISKS: - High Dose of Corticosteroids - Chemotherapy - Granulocytopenia Clinical Features: - Starts with invasie pulmonary infection -- Fever, cough, chest pain - Dissemination to other organs -- Brain, Skin and Bone - Grnulocytosis resolution impacts patient outcome -- If granulocytopenia persists, mortality is high -- With Cerebral abscess mortality is 100% Treatment - Invasive aspergillosis is treated with several weeks of IV amphotericin B - Itraconazole or voriconazole can also be used as supplement. |
|
|
Describe Hyalophyphomycoses:
|
- Mycotic infection caused by a number of hyaline (non-dematiacous) Hyphomycetes.
- Etiologic agents in this group include: -- Penicillum, Paecilomyces, Acremonium, Beauvaria, Fusarium, Scopulariopsis and Pseudallescheria Symptoms: - They can cause systemic infection including CNS in immunocompramised patients - Can cause brain infection after traumatic injury. Phaeohyphomycosis: - Most common Cladosporiosis: -- are also known as strong "aero-allergens" and sometimes "toxic mold" - Organisms found everywhere in nature and are associated with decaying plant and trees. - Spores survive for long periodes in the soil Etiology: - Group of mycotic infections characterized by the presence of dermaticeous (dark-walled) septate hyphae and yeast or combination of both in the tissue Symptoms: - Infections in deep systemic tissues -- Skin, muscle, CNS - CNS infections tend to occur in person on chronic peritoneal dialysis or that have had traumatic head injury - Infection of CNS with this organisms has POOR progrnosis Diagnosis: - Colonies around 3 cm in 7 days on PDA, olivaceous grey-green olivaceous brown, reverse olivaceous black. Diagnosis: - organisms grows very slowly in culture - Histopathology of infected tissues where dark mold and yeastlike forms may be identified Treatment and Prevention: - Food Products: -- Wsh with hot chlorinated water -- Fungicides to surfaces |
|
|
What is Phaeohyphymycosis?
|
MOST COMMON cladosporin.
- Are known as strong "aero-allergens" and sometimes "toxic mold" - Organisms found everywhere in nature and are associated with decaying plants and trees - Sores survivie for long periods in the soil Etiology: - Group of mycotix infections characterized by the presence of dermatiaceous (dark walled) sepate hyphae and yeast or a combination of both in the tissue. Symptoms: - Infection in deep systemic tissues --- skin, muscle, CNS - CNS infection tend to occur in persons on chronic peritoneal dialysis or that had traumatic head injury - Infections of CNS with this organisms have POOR prognosis. Diagnosis: - Colonies around 3 cm in 7 days on PDA (Dialysis), olivaceous black. Diganosis: - Organisms grows very slowly in culture - Histopathalogy of infected tissues where dark mold and yeastlike forms may be identified. Treatment and prevention: - Food Products -- Wash with hot cholirnaed water Fungicides to surfaces. ** KEY IS TO PREVENT IT** |
|
|
Describe the Protozoa Toxoplasma Gondii?
|
- Toxoplasmosis - zoonotic protozoa gound in mammals and birds.
- Congenital Infections is caused by transplacental transmission to the fetaus - Opportunistic infection (HIV) as encephalitis Diagnosis: - Examination of anti-toxoplasma IgG antibody in serum - Brain is most common sites of infection and necrotizion often occurs. |
|
|
Describe Treponema Palladium ?
|
- Early stages have no CNS symptos
-- Tertiary Syphillis -- Takes 10 years or long -- No longer infectious -- Low titers of the organisms Pathophysiology: - Delayed hypersensitivity is part of the pathologic mechanism of tissue damage in theis tertiary stage. - Common lesions found in various tissues called "gummatous" lesions. - Infection of CNS ia meningovascular syphillis. Symptoms: - CNS degenerative changes = mental changes - May have frank psychosis - Shuffling gain "tabes dorsalis" Diagnosis: - Spinal fluid may be helpful -- VDRL positive -- Elevated WBCs -- Elevated Proteins Treatment: - Depends on the stage of the disease - Later stages will necessitate longer regiment of treatment (~30 days) - Penicillin - Doxycycline for Ppenecillin allergies. |
|
|
Describe Leptospira Interrogans:
|
- Spirochete endemic in animals
- Transmitted through animal urine contamination of water and food (can survive weeks in the water) - No natural body of water in the US can be expected to be free of leptospira - Acidic solution and drying and SOAP will KILL IT. Epidimiology: - Incidnce usually <100 cases per year in US. - Populations at risk: -- Sewer worksers -- Miners -- Veternarians -- Meat packers Symptims: -- Incubation 7-13 days althought rangers 5 days - 4 weeks. - Bacteremic Phase - influennza like symptoms and fever (spirochetes NOW enter the brain). - 2nd phase - 3 + weeks - Headache with "aseptic" meningitis - Sometimes hemodynamic collapse Diagnosis: - Blood culture - CSF analysis and culture -- Raise in antibody between acute and convalescen stages Treatment: - Antibiotics shold be started within 4 days of the bacteremic phase to be effective -- Penecillin -- Tetracyclin |
|
|
Describe Borrelia Burgdorferi - Lymes Disease
|
- 15% Neurologic Abnormalities
- Rarely Fatal - Starts with a tick bite (Ixodes) - Large Spirochete - 0.2 x 10-30 um Symptoms: - Classic "bulls eye" rash - Constituional Symptoms - Fever - MS and joint pains - Meningeal irritation symptoms 2nd stage symptoms: - Dissemination system wide 3rd stage symptoms: - Mild neurogic or frank encephalitis Diagnosis: - Loose irregular spirals - Silver and special immunoflourescent stains - Culture is difficult and not often used clinically - CDC (1999) recommends antibody screen using ELISA Treatment: - Early antibiotics -- Doxycycline daily x 14-28 days - Late Disease (given over several weeks) --- Parenteral penicillin OR ceftiaxone - Avoid Bismacine (often given by naturopaths .. may cause heart and renal failure). |
|
|
What is Transmissible Spongiform Encephalopathies?
|
- NOT a virus
- Is an infectious protein called a PRION: -- Prion protein is an abnormal isoform of a normal encoded protein. -- Prions have NO nucleic acid of any kind -- Replicate w/out prevoking an antibody response -- Are resistant to conventional inactivation methods for bacteria and viruses. A proteinacouse infectious particle. A normapl protein that has a change in its three dimentional configuration. As the prion attacks the brain it causes large vaculouse in the cortex and cerebellum, giving a spongiform appearence. Prion alternation of protein: -- Normal (PrPc) - glycoprotein with secondary structures dominated by alpha helixes. -- Prion Protein (PrPSc) glycoprotein with 2ndry structures dominated by beta helices -- When PrPSc molecules come in contact with PrPc molecules, they are converted to PrPSc, and aggregates from resulting in vacuole formation. |
|
|
What is the Creutzfeld Jakb Disease?
|
- Most COMMON prion in Humans
- 1:1,000,000 per year - Peak incidence 55-65 y/o, but has affected teenagers - No effective treatment - CJD is a prion and is NOT JC virus which is a virus. Symptoms: - Insidious mental deterioation -- Early cerebrallar and visual problems -- Severe dementia in 6 months -- Brain and lower motor neuron inolvement -- Cases of CJD have been due to: ---- infected corneal transplants ---- Reused brain equipment not steralized --- Pituitary hormone injection derived from cadavers --- Acidental cuts during autopsies / surgeries |
|
|
Describe Spongiform Encephalities:
|
- BSE returned to the new in 1996
- Variant form of CJD - Both are progressive neurodegenrative disease resulting in patient death. |
|
|
Describe Bovine Variant of CJD?
|
- Degenerative Fatal Brain Disorder
-- Normally Bovine but crossed to humans --- Mad Cow Disease -- Caused by feeding cattle protein enriched feed not processed correctly vCDJ diagnosis: - Biopsy of brain -- Spongiorm Encephalopathy -- Accumulation of abnormal folded proteins - Sporadic Disease - CSF: no cells - New test identifies specific proteins (14-3-3) but not as yet definitive. vCDJ Treatment: - No drugs, no immunization - Prevention - avoid cutting yourself - Eat fish and get brain disease from mercury. |
|
|
In a funcal CNS infection, analysis of CSF would reveal?
|
- Normal pressure, WBCs < 100, Normal protein, lymphocytes.
- Variable Pressure, var. WBCs, elevated protein, lymphocytes - Elevated pressure, > 1000 WBCs, mild to high protein, PMNs |
|
|
What is the most common etiology of fungal menigitis:
|
- Aspergillus Species.
- Cryptococcus Neoformans - Histoplamsa Capsulatum - Candida Albicans |
|
|
Describe the structure of Hepatovirus A (HAV)
|
- small naked virus with icosahedral capsid symmetry.
- Previously this virus was called, Enterovirus - 72 and Hepatitis Virus A. - ss Positive RNA - very stable under normal physiological conditions. - It cause inectious hepatitis - Stable to many physical factors Resistant to: - Stomach acid (pH 1) - Drying, heat and salt water - Solvents and detergents Sensitive to: - Chorine and formalin - Ultraviolet Light Hepatovirus A: - Naked, icosahedral Virus - Viral Protein (VPg) is attached to the 5' end of Single - Stranded Positive Sense RNA. |
|
|
Whats the major route of transmission for the hepatovirus A?
|
- Oro-Fecal
- also raw undercooked shellfish, oysters and clams. - When virus is shed in feces, it can be sexually transmitted. - During Viramic stage, you can shed in blood. KEY IS: ORO-FECAL spread Incubation Period Is: 15-40 days |
|
|
Describe the time course of Hepatovirus A infection?
|
- Virus Shedding in the feces BEFORE the symptoms appear
- Shedding stops before the cessasion of symptoms - 2 weeks viremia with possibility of blood infection Transmission: - Blood Products - Anal Sex - Body Secretions |
|
|
Hepatovirus A replicates in?
|
- Kupfer's Cells causing symptoms such as:
-- Fever, Nausea, Vomiting and Jaundice -- Recovery is uually complete within 8 - 12 weeks. |
|
|
How can we control Hepatovirus A?
|
- Prevent Fecal Contamination of food and water w/ popor senation techniques.
- Areosol transmission is rare, but it is important to contain aerosols. |
|
|
Describe the Hepatovirus B structure:
|
Infectious virus is called DAN particles. Found in patients Serum.
Partially DS DNA Viral DNA polymerase serves as the reverse transcriptase. |
|
|
For Hepatovirus B what is the most useful inicated?
|
Viral Surface Antigen (HBsAg) found in the CORE.
|
|
|
How is Hepatovirus B spread?
|
Usually throught the transfussion of contaminated bloor or blood products.
Oral and sexual transmission is very common as the viron is detected in ALL body fluid. Chronic carriers (IV drug abusers) are the major source of contamination. Another major way of spread is Iatrogenic and Nosocomial (via surgery) |
|
|
Describe Epidimiology and Pathogenesis of Hep B?
|
During latter half of the incubation period, urine, semen, vaginal secretions, breast milk feces and nasopharyngeal secretions all contain HBsAg.
Symptoms: fever, rash and arthritis begin insidiously with variation in severity. Mild cases are an-icteric. The disease is rarely over 8 - 10 weeks. Overall mortality ranges from 1% - 2%. |
|
|
Describe how a chronic / acute Hep B develops and what do they each have?
|
Acute Disease develops from a CMI: and it is due to cell damage:
- Jaundice - Dark Urine - Pale Stool - Nausea Leads to recovery Chronic Disease develops because of Limited CMI and can lead to: - Fulminant Hepatitis - Cirrhosis - Primary Hepatocellular Carcinoma |
|
|
Describe the replication of HBV:
|
1. The virus core enters the hepatocyte leaving the coat out side
2. Viral DNA is released from the core by celular enzymes 3. Viral enzymes fill the gap of the "partial" ds DNA into complete circular ds DNA which moves into the Ncleus of the hepatocyte. 4. Transcription of DNA into many species of mRNAs and one full lengh pre-genmoic RNA is also transcribed. 5. ALL mRNAs and the pregenmoic RNA now move into the cytoplasm of the cell 6. The mRNAs translate the viral structural protein (coat, core) and nonstructural proteins (viral enzymes including reverse transcriptase) 7. Viral core get assembled by the structural proteins and pregenmoic RNA long with the reverse transcriptase (RT) enters the core. 8. Within the core, the RT recognizes the pregenmoic RNA and transcribes it into the DNA converting it into RNA-DNA hybrid 9. RNASE H activity of RT digest the RNA part of the hybrid leaving a few nucleotides undigested as a primer (due to confinement within the core) 10. Now DNA Polymerase activity on the RT polymerizes the second complementary strand of the DNA on the ssDNA primer, making it partially ds DNA. 11. This is due to lack of any space for the RT to complete the two strands of DNA within the confinements of the core. 12.While the transcription if taking place, the core gains its coat and moved towards the cell membrane to mature into a complete virus. |
|
|
Describe the Infection Cycle of HV
|
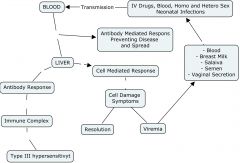
|
|
|
What are sme unique features of Hepadnaviridae?
|
- Enveloped with a protein coat (no lipid bi-layer membrane envelope).
- Partially ds circular DNA - Replication of the viral genome is via circular DNA intermediate - A DNA containing virus requires a virus associated Reverse Transciptase. - Both Surface and CORE antigens of the virus share sequences but hae different inframe start codons. - The viruse shows strict tissue tropism for the liver cells - Genome can integrate into the host chromosome during chronic state and cause liver cancers - Infected liver cells release HBsAg particles which lack viral DNA. |
|
|
What are the viral antigens?
|
- Surface Antigen (HBsAg)
-- Hepatitis Associated Ag (HAA) - It is detected in laege quantities in serum infected individuals and is pleomorpic in shape under the EM. Most Useful Marker There is Group Speciic Epitope: [A] And a type specific epitope: [d,y,w,r] Soluble Antigen [HBeAg] - Indicator of SERUM infectivity - The pre-core protein cleves into HBeAg and HBcAg proteins. It does not assemble in the infected cells but is secreted into the serum. Core Antigen (HBcAg): - It is observed in Infected hepatocytes and is not found free in serum. Te core protein (HBcAg) has protein kinase activity. |
|
|
Describe the events that occur during Hepatitis B Acute Infections:
-- Know what the window period is! |
- Viremia starts 1 month before symptoms
- HBs-Ag and HBe-Ag in serum - Anti-HBc-Ab 1st antibody to appear - Window Period - no HBs-Ag or Anti-HBs Ab detected. |
|
|
Describe the Development of Chonic HBV carrier state:
Describe the viral persistance: |
- Viremia countinues for years
- Both HBs-Ag and HBe-Ag are detected for years - Anti-HBc-Ab always present - No Window Period Viral Persistance: - 5-10% of cases may continue to have HBsAg in their serum for life -8-10% also carry high concentration of Dane Particles - All carriers have anti-HBcAg and some anti-HBeAg. Those w/out anti-HBeAg often have high levels of circulating HBeAg. |
|
|
Persistance infection of HBV (Hepatits B Virus) correlate with:
|
- Hepatocellular Carcinoma
- Tumors cells obtained from liver contain HBV-DNA - Percise mechanism of oncogenesis is not known as the HBV does not carry any oncogenes. - Perhaps the virus is involved in an insertional or transactivating mechanisms: |
|
|
How do you control HBV infections?
|
- Screening of all donated blood for HBsAg must be regularly conducted.
- Both inacticated (Heptavax) and recombinant subunit HBsAg (recombivax) vaccines availible for high risk individuals. |
|
|
What are the common features of Retro- and Hepadnaviridae?
|
- Both families use RNA to make DNA using virus associated Reverse Transciptase activity.
- Chronic Infections of cells results in cell destruction - Order of functional genes in both families: --- Retro: gag-pol-env --- hepadna: C - P - V Both viral families can cause some cancers. |
|
|
Describe the Hepatitis C Virus?
What is the predictor of the disease? |
Structure:
Enveloped viruse, 40-60nm in diameter, cosahedral nucleocapsid contains ss positive RNA Classified as a Flavivirus. Epidimiology and Pathogensis: - Liver infections which did not prove to be Hepatitis A or B were designed as NANB infections. Now these test postive for HCV. Transmission is by sexual and IV drug use. Incubation period ranges from 40 to 120 days. Predictor of disease is high Alanine Amino Transfarase Half of the post transfusion patients develop chronic liver disease and many of these develop cirrhosis or hepatocellular carcinoma. - Infects humans and chimps - The virus coats itself with LD and VLD lipoproteins to utelize the hepatocyte cell receptor to enter the cell. CD81 and other lymphocytes are suseptible to the virus The virus buds into the ER and remains there. After entery into the hepatocyte, it inhibits apoptosis and INF-alpha, by binding to TNF receptor and protein kinase R which establishes persistence (no cell death). Continuouse liver repair causes cirrhosis and predisposing to primary hepatocellular carcinoma. |
|
|
What is the outcome of Acute Hep C infection?
What about Chronic? |
- 15% complete recovery
- 15% Cirrhosis - 70% Persistance Infection Persistant infections leads to Chronic Infection and Asymptomatic Infections. Chronic Infection can lead to: - 6% Liver Failure - 4% Hepatocellular Carcinoma - 20% into Cirrhosis |
|
|
How do you control HCV?
|
- No vaccine are avalible
- Presecreening of blood donors and products is now required. |
|
|
What do you use to treat HCV?
|
- PEGylted Interferon Alpha has shown promise.
|
|
|
Describe the Hepatitis D virus? HDV?
|
- Infection is thru sexual contact, blood products and among IVDA. Vertical Transmission is Possible.
- Epidimiology is similar to HBV, Simultanous infection of HBV and HDV results in mild infections. HDV infection subsequent to HBV infection results in rapid and severe hepatitis. An infection by delta virus in populations with chronic HBV ocurs with progressive liver disease with up to 20% mortality. Diagnosis is by detection of anti-delta IgM and/or IgG. Control: - Control of HBV will control HDV possibly by accinating the high risk population againt HBV. |
|
|
Describe the viral structure of Hepatitis D Virus:
|
- Enveloped viral agent, 35-40 n in diameter containing ROD SHAPED, SS NEGATIVE circular RNA which only codes for one protein. DELTA ANTIGEN.
REQUIRES presence of replicating HBV which provides the viral coat ( containing HBsAg in addition to delta antigen). |
|
|
Describe Hepatitis E virus?
|
Small icosahedral naked virus, 32 - 34 nm in diameter , contains ss positie RNA.
Types 3 and 4: human and swine (common in older and immunocompromised patients). Type 5: birds Transmitted mortality among fecally contaminated water. 20% mortality among pregnant women Outbreaks through out the world involving thousands of people Transmitted to non-hman primates through human feces and recovered from infected animals. Control: Better Hygine, controlled water and sewage treatment. No Vaccine Available |
|
|
Describe Hepatitis F Virus?
|
- Viral Structure:
-- Enveloped Virus w/ Icosahedral Nucelopcapsid containing ss positive RNA, 60 nm in diameter, Togavirus-like agent. Epidemiology and Pathogenesis: - Oro-Fecal transmision - May cause malignancy and rejection / necrosis of grafted liver. Control: There are no vaccine but you can use water and sewage treatment. |
|
|
Describe Hepatitis G Virus (HGA)
|
Enveloped Virus containing ss positive RNA (Flaviviridae)
Epidemiology and Pathogenesis: - Transmissible to Chimpanzees - CAUSES syncytial giant cell hepatitis Control: - no vaccines, source of infection is NOT known. |
|
|
Describe the TT-Virus Hepatitis:
|
Cosahedral, nakes virus containins ss with negative polarity and CIRCULAR DNA and classified as:
- Family: Circinoviridae - Genus: Circovirus - Virus: TT-Virus Epidemiology and Pathogenesis: - Isolated from NON-A non-G hepatitis sera in 1997. - 12% of Japanese and 60-70% of Norwegians have anti-viral antibodies. There are no vaccines. |
|
|
What are the viral infections of the liver?
And what about the Hepatits in Neonates or Immunocompramised. |
- Hepatitis Virus A-G
- TT viruse hepatitis (NON A - NON G hepatitis) - Yellow Fever Virus - Epstein-Barr Virus Hepatitis in the Neonate or Immunocompraised: - Human Herpes Virus 5 (Cytomegalovirus) - Human Herpes Virus 1 (Herpes Simplex Virus 1) - Humn Herpes Virus 3 (Varicella Virus) - Rubella (Congenital Rubella Syndrome) |
|
|
What virus causes the infections of:
1. Heart 2. Kidney 3. Muscle 4. Eye 5. Glands |
1. Coxsackie B Virus
2. Cytomegalovirus 3. Coxsackie B Virus (Pleurodynia) 4. - Human Herpes Virus 1 - Adenovirus (MOST COMMON) - Rubeola (Measles) and Rubella - Enterovirus 70 - Coxsacke A24 Virus 5. - Human Herpes Virus 5 (Cytomegalovirus) - Mumps |
|
|
Describe the spread of the MUMPS virus:?
|
Replication: Epithelial Cells of Respiratory Tract.
Patients infectious before any symptoms, virus shed in saliva and rine up to a month. The Virus gets into the blood. IF the person is NON-Vaccinated: - Testes / Ovaries: Orchitis is the first symptoms, 15 day PI. Parotid Glands: Infects ductal Epithelium, Swelling principle symptom, 15-17 day PI Eyes, Inner Ear, peripheral nerves CNS IF the person is Vaccinated: Ab: no value intracellular virus-> CMI: essensial cause of symptoms -> Resolution. See page 18 Lecture 43-45 |
|
|
Describe the time sequence of MUMPS infection:
1. When can you recover the virus from urine? 2. When do you see Orchitis? Prrotitis? Meningeonecpehalits? 3. When can you recover the virus from CSF? 4. When is the virus specific antibody is present? |
1. 15 - 29 days
2. Orcitis: 15-29 days/ Parotitis - 17-30 days, Meningoencephalitis - 29-50 days. 3. 29 days 4. 23 days |
|
|
Describe the Betaherpesvirinae?
|
- Long viral replication cycle
- Infected cells, swollen (cytomegaly) - Latency in glands, kidney and liver Cytomegalovirus (HHV-5) - Most cytomegalovirus (CMV) infections are sub-clinical. Spread through the contact with infected urine, saliva and breast milk. Virus is also found in semen and genital secretions of the infected individuals. Infected children shed virus for a long time. Epithelial cells in the oropharynx are the primary target. Typically giant cells with Cowdry Type A inclusion bodies seen in salivary glands, spreading to lymphoid tissue and viremia. ~50% babies found infected when mothers had a primary infection during pregnancy. Latent Infections: - Life long latent infections, virus shed in saliva and urine for months to years after primary infection. |
|
|
Describe the major clinical syndromes of Cytomeegalovirus (HHV5) Infection?
How do you diagnose it? |
Congenital Infections:
- About 20% of all infants with congeinital CMV are symptomatic with jaundice, microencephaly, hepato-spleenomegaly and lethargy. The asymptomatic infants develop viriuria within 1 week after birth and continue to shed the virus. Perinatal CMV: - Vast majority of infections are asymptomatic - Pneumonitis may be seen occasionally during the first three months. CMV in the immunocompromised: - Primary infections or reactivation of the latent virus occurs within 2 months of transplantation. Leukemia and lymphoma patients are at high risk. CMV retinitis, colitis and pneumonia are common in AIDS. Diagnosis: - Cytomegalic cells (Owl Eye) - Virus isolation from salaiva and urine - RIA and ELISA |
|
|
What is the treatment of Cytomegalovirus (HHV5)?
|
Gancyclovir - acyclyc guanosine analogue, decreased virus shedding in all patients.
Acyclovir - resistant to acyclovir. NO VIRAL THYMIDINE KINASE. Human Leukocyte Interferon delays virus shedding. |
|
|
describe the Adenoviridae?
|
- Icosohedral, naked virus resistant to ETHER, contains double stranded, linear DNA with terminal repeats. A 55K protein at 5' end on each strand.
IMPORTANT ASPECTS: Pentone Fiber: Toxic to the cell, has HA activity and important cell attachment. VA-RNA: Early RNA, blocks interferon induction in the neighboring cells. Persistence: Lymphoid tissue (healthy tonsils, Adenods). Continuous slow virus release from the lymphoid tissue. Diagnosis: Nuclear inclusion bodies in infected cells. Treatment: Rifampin, results in immune virus. |
|
|
Describe the epidemiology of the adenovirus?
|
- More then 120 serotypes, only 51 effect humans.
- Divided into groups A - F according to group specific antigen situated on the HEXON capsomeres. These antigens are the CF antigens. Serotypic antigens are detected on the basis of HA activity which is located on the Penton Fibers. The virus persists in the host for days to years. The virus is isolated from the tonsils, nasopharynx and intestinal tract of apparently healthy individuals. Oro-Fecal and respiratory tract spread during childhood. About 45% of infections result in disease. Clinical Aspect: Repiratory: - Fever, Rhinitis, Cough and exudative pharyngitis under 3 years of age. Acute and Chronic conjunctivitis, laryngitis, croup, bronchitis, pneumonia and Pharyngocojuctivitis. Hemorrhagic Cystitis: Hemeturia Gatroenteritis |
|
|
Describe the pathogenesis of Adenovirs?
|
Direct inoculation of the virus into nasal or conjuctival mucosa by hands or towels. Virus replicates in the epithelial cells producing cell necrosis and inflammation. Virimia soetimes spreads into lymphoid tissue, kidney and bladder.
Persistence: Lymphoid Tissue: - Tonsils - Adenoids - Peyer's Patches Reactivation of the virus takes place thought STRESS and OTHER infections. PATHOLOGY: - epithelial cell necrosis is seen with mononuclear inflammatory response. In some cases intranuclear inclusions may be seen (Cowdry Type A) Immunity: - Long Lasting and Serospecific - Group Specific CF antibodies Diagnosis: - Virus isolation in cell cultures - Testing paired sera for 4 fold increase in titer Prevention: - Inactivation vaccine ( serotypes 3,4, and 7) - Live vaccine recommended for military recruits. Treatment: - Rifampin results in the release of immature virus |
|
|
When does bacterial colonisation of the GI tract begins?
|
AT BIRTH: initial inoculum comes from the mother's vagina and external genitalia.
In the first 2 weeks: colonisation and adjustment of colon takes place, initially: E. Coli and Streptococcus (10^8-10^9 org/g feces) is present. In 4-7 days: Bifidobacterium #s will increase (breast feeding). Then Clostridium & Bacteroides #'s are high. |
|
|
What is the difference between breast fed and formula fed babies/feces?
|
Formula-Fed contains labctobacilli in feces: metabolize sugars.
Breast-Fed contains Bididobacteria in feces: metabolize proteins. Feeding type results in differenced in consistency and color of the stool. There is a research correlation with later life obesity due to the suger content of formula's hence formula companies are now changing composition of formula milk (less sugers). It takes about 2 years for infant colon flora to resemble adult flora (10^10 - 10^12 / g) = after weaning onto solid food. |
|
|
Describe the bacterial environment in the MOUTH:
|
- Microbial count > 500 different bacterial species (predominantly anaerobes and faculataive's ) in the biofilm attached to surface of teeth and gums.
- There are also some fungi and transient viruses (mumps, cytomegalovirus and coxsackievirus) |
|
|
Describe the Flora of the STOMACH:
|
- Parietal cells release HCL, Gastric pH is around 3.
- Chief Cells secrete and release pepsinogen. MICROBIAL count is usually sterile (0 bacteria / ml) H. Pylori may be isolated in gastritis. NOTE: if stomach microbial counts are in the region of 10^5 or 10^7, this indicates an abnormality. E.G. achlorhydria or malabsorption syndrome. |
|
|
Describe the LIVER, function and bacteria?
|
LIVER:
- Release BILE ( a steroid with detergent properties ). Begins digestion of fats. |
|
|
Describe the Pancreas, function and bacteria?
|
- Pancreatic juice, Sodium Bicarbonate (NaHCO3) neutralizes the acidity from the stomach (pH 8). Enzymes:
- Amylase - Lipase - Zymogens (trypsin, chemotrypsin, elastase, carboxypeptidase) and nuclease. |
|
|
Bacterial numbers _________ the further you go from the stomach...
|
Increase
|
|
|
Describe the function/bacterial composition of the small intestine:
|
- Site of absorbption of most neutriants, surgers and amino acids, fats plus ~ 90% of salts and water.
|
|
|
Describe the Duodenum, function and bacterial composition:
|
- Microbial counts increase (0-10^4.5 bacteria/ml). Tends to be fluctuating transients. e.g. aerobic Streptococci, Staphylococci, Streptococci, Lactobacilli, yeasts, anaerobic Streptococci and Lactobacilli.
NOTE: the complete absence of coliforms and bacteroids in this area. |
|
|
Describe the Jejunim-Ileum, function and bacterial composition:
|
- Microbial counts increase to 0-10^5 - 10^7 bacteria/ml.
e.g. - Enterobacteria and some - Streptococcus - Staphylococcus - Lactobacillus - Bacteroides - Bifidobacterium - Clostridium NOTE: Start to isolate coliforms and bacteroids in this area. NOTE: at the ileocaecal junction #'s increase dramatically to 10^6 - 10^8 organisms/ml. |
|
|
Describe the large intestine (LI)/Colon, function and bacterial composition:
|
Completes absorption (water and remaining salts). Microbial count are the highest at 10^10 - 10^12 bacteria/ml comprising of > 400 species. 95-99% are anaerobic.
e.g. - Bacteroides - Bifidobacterium, - Clostridium - Eubacterium - Peptostreptococcus species - With MINORITY being members of the enterobacteriacea (facultative anaerobes). |
|
|
Describe the ALLOGENIC [originating from outside of the GI]factors affecting the microbial composition of the GI tract.
|
a. Diet - the maeal can influence gastric empetying, having an effect on bacterial composition.
Western Died: Increase the # of Bacteroides, Decrease Enterococci (or other aerobes). Vegetarian Diet: decrease #'s of Bacteroides, increase #'s of aerobes. b. Age - Could be due to diet, in terms of quantity and type of food or immune status. c. Geographic Location: Compare Western vs. Eastern Societies. d. Antibiotic Therapy - Causes distirbuances or Removal of normal flora within GI tract. Results in increased susceptibility to colonization by pathogenic microbes. e. Surgery - Research demonstrates an alteration of the bacterial population at a particular location. |
|
|
Describe AUTOGENIC [those thst arise from the GI ecosystem itself]
|
Enviornment:
Think about the GI tract enviornment conditions: 37C, anaerobic. conditions as you move down the GI tract. -- Hydrogen ion concentration (i.e. gastric acid): influences colonization of the stomach. -- Peristalasis (directory of flow): influences colonization of the stomach. -- Shedding of the epithilium: prevents colonization stability. -- Mucus: Antimicrobial -- Conjugated Bile Salts (some studies investigated). -- Immunological Response (IgA) b.) Activities of Microorganisms: -- Nutritional and Attachment site: competition for -- Produce bacterial inhibitors: bacteriocins, antibiotics (normal 2nd metabolic products). -- H2S production -- Maintence of low-oxidation-reduction potentials. |
|
|
What are some ORAL Diseases?
|
Dental Caries and Periodontal Disease (gingivitis, periodontis) are common chronic oral disease worldwide.
Dental Caries: Infectious diseases resulting in localized dissolution and destruction of the calcified tissues of the teeth (enamel, dentin or cementum). Diverse Microbes Involved: - S. Mutans - S. Sanguis - Lactobacilli - Actinomyces Naeslundii - A. Odontolyticus - Propinobacterium Species. - Eubacterium Species - Fusobacterium Species - Capnocytophage Species - Viellenola Species Prevention: - Fissue Sealents - Fluoride - Antimicrobial Agents - Vaccination |
|
|
Describe Peridontal Disease:
|
Infections affecting supporting structures of the teeth (gingiva, cementum, periodntal membrane and alveolar bone). Gingivitis: inflammation of the gums. Is reversible BUT if untreated can progress to periodontis.
|
|
|
Describe Abscesses
|
In mouth, leading to deep tissue (alveolar bone, lung brain or extermities.)
An abscess (Latin: abscessus) is a collection of pus (dead neutrophils) that has accumulated in a cavity formed by the tissue on the basis of an infectious process (usually caused by bacteria or parasites) or other foreign materials (e.g. splinters, bullet wounds, or injecting needles). It is a defensive reaction of the tissue to prevent the spread of infectious materials to other parts of the body. |
|
|
Describe the most common bacterial pathogens associated with ingestion of food?
2. Stool samples determine what? 3. When do you test for EHEC? |
1. Campylobacter Jejuni
2. Salmonella Species 3. Shingella Species 4. E. Coli O157:H7 5. Yersinia Enterocylytica 2. Campyllobacter, Salmonella and Shingella Species. 3. EHEC is only screened for if the speciment is BLOODY |
|
|
Describe the FOOD-BORNE infection DIARRHOEAL:
- Bacillus Cerus in terms of: 0. Describe the bacteria 1. Onset of Duration 2. Principle Symptoms 3. Typical Foods 4. Mode of Contamination 5. Pathogenesis: |
0.Gram +ve (0.7 um by 3-10um long) arranged in chains. Aerobic or Facultative. Spore Formain.
1. 8 - 16 hours (12-24 hours) 2. Watery Diarrhoea, Cramps, Occasional Vomiting 3. Meats, Soups, Sauces, Vegitables. 4. From Soil/Dust 5. Produces ST Neurotoxin |
|
|
Describe the FOOD-POISONING infection EMETIC:
- Bacillus Cerus in terms of: 0. Describe the bacteria 1. Onset of Duration 2. Principle Symptoms 3. Typical Foods 4. Mode of Contamination 5. Pathogenesis: |
0. See the prev. slide
1. 1-5 hours typical is <4 hrs, (6-24 hours) 2. Nausea, vomitting, sometimes diarrhoea and cramps. 3. Cooked Rice and Sometimes Pasta 4. From soil/dust 5. Produce ST Neurotoxin. To culture use non-selective medium, i.e. blood agar (plus polymyxin to supress gram -ve). Food should contain >10^5 organisms/gram. |
|
|
Describe the FOOD-POISONING infection Botulism:
- Clostridum Botulinum in terms of: 0. Describe the bacteria 1. Onset of Duration 2. Principle Symptoms 3. Typical Foods 4. Mode of Contamination 5. Pathogenesis: |
0. Variable in size, +ve gram, Anaerobic, Ferment a range of CH2O -> Gas: Spore former, produces exotoxins, Susceptible to Penecillin.
Charachtarized by: Descending Systemic Paralysis: rarely seen, more common in children. 3 Types of Disease: - Food Poisoning: injestion from pre-formed toxin in food - Wound Botulism: Rare, organism grow in necrotic tissue of wound (NO GI disturbances). - Infant Botulism: Ingestion of spores, organisms grows and produces the toxin once in the infant intestines. 1.12-36 hours 2. Fatigue, Weakness, Double Vision, Slurred Speech, Respiratory Failure, Sometimes Death. Also: 1/3 patients show GI desturbances in A and B type and ALL patiens in E type. Other symptoms: nausea, vomiting and abdominal pain, diarrhoea, constipation may occur. 3. Types A and B: home canning of fish, fruits, vegitables, condiments. Type E: Fish 4. Type A and B: from soil or dust and Type E: water and sediments. 5. Produces 1 or 8 exotoxins (A, B, C1, C2, D, E, F, G). Human infection is due to A, B E and rarely F. Animal Infection is mainy from C and D. In USA the most frequent toxin isolate is A then B and E. In Europe the most frequent toxin isolate is B (A is rare). Exotoxin: Botulinum toxin, is a protein Neurotoxin, it prevents the release of acetylcholine across synapse gaps. Infective Dose: Type A is the most potent. |
|
|
Describe Botulism (Infant)
1. Onset 2. Principle Symptoms 3. Typical Foods 4. Mode of Contamination 5. Pathogenesis |
It is NOT a food poisoning. Affects infants between 2 weeks and 6 months.
1. N.A 2. Constipation, Weakness, Repiratory Failure, Sometimes Death, Child sleeps more then normal, suck and gag reflex diminish, Dysphagia becomes evident as drooling, Later: Head control is lost and infant becomes flaccid. In Severely infected individuals infection can lead to respiratory arrest (4-15% cases result in sudden death). 3. Dust (assciated with honey) 4. Ingested Spores from soil/dust 5. Ingestion of spores --> Germination in GI tract --> Vegetative Cell Replicate --> Release toxin in intestine (implicated TYPES A and B) Treatment: Lg. dose of horse C. Botulinum Antitoxin + Supportive Measures. Botulism Immune Globulin Intravenous (human) (BIG-IV). |
|
|
Describe the Diagnosis of botulism?
|
NOTE: this is a reportable disease, it is a fatal food toxemia, so it is important to rule it out from your diagnosis.
-- Presumptive Diagnosis --- Presence of rapidly descending paralysys --- History of Ingestion of home canned food -- Confirmative Diagnosis --- Demonstration of botulinium toxin in serum/faeces or incriminating food (via mouse-toxin neutralizing test). |
|
|
When Diagnosing for botulism, what should my differential diagnosis include?
|
- Guillian-Barre Syndrome
This is an ascending paralysis. Paresthesia or other sensory abnormalities, elevated cerebral spinal fluid protein. Myasthenia Gravis: a descending paralysis. Accentuation of muscle fatigability during exercise and positive response to endrophomium. Other microbial food posonings and gastroenteritis: NO cranial nerve involvement Chemical (non-microbial) food poisoning: Symptoms occur within minutes. |
|
|
Describe the two types of Mushroom Infection:
|
Cause Food Poisoning:
Short Acting or Long Acting: Short Acting: Variety of toxins: museinol, muscarine, psilocybin, cprius, artemetaris, ibotenic acid (Neurotoxins). Incubation: Quick Onset < 2 hours. Symptoms: Generally Acute, Predominantly Severe Nausea, Vomiting, Diarrhoea. Long Acting: Amantia. Incubation: 4-8 hours. Symptoms. Diarrhoea, Abdominal Cramps Can be FATAL. |
|
|
Describe what are MYCOTOXINS and AFLATOXINS:
|
MYCOTOXINS: They are secondary metabolites that develop on/in food primarily during poor storage. Produced by variety of fungi: Aspergillus, Fusarium and Penicillium
Aflatoxins: Produced by Aspergillus Flavus and A. Parasiticus. Fungal Growth on Tree Nuts, Peanuts, Oil Seeds (corn and Cottonseed). Result of Ingestion. Acute Necrosis, Cirrhosis and Carcinoma (Liver). |
|
|
What is Ciguatera Posoning?
|
It is associated with ingestion of contaminated fish.
Pathogensis: Large predatory reef fish eat dinoflagellate algae Gambierdiscus toxicus. Dinoflagellates release Ciguatoxin (a Neurotoxin) in fish. Bioaccumulation of toxin in fish liver and viscera. Cooking does not denature/remove the toxin. Clinical Symptoms: Incubation: min - 30 hours, typical 3-6 h after ingetion. Duration is 1-2 weeks. Watery Diarrhoea, nausea, adbominal cramps (after 12 hours), muscle aches, burning sensation. Neurologic symptoms: circumoral & extremity paresthesia, severe pruritus, hot/cold temp reversal. |
|
|
What is Scromboid Poisoning:
|
Also known as: Non-Allergic Histamine Fish Poisonin.
Associated with ingestion of contaminated Scrombridae Fish. e.g. tuna, mackerel, mahi mahi, marlin, bluefin and bonito. Pathogenesis: Histadine normally is present in fish. Bacteria is in/on fish (e.g. Stenotrophomonas maltophilia, Morganella Morgani) convert Histidine --> Histamine (also known as Scrombotoxin). Results in Histamine accumulaion in fish. Cannot be eliminated by cooking, freezing, salting etc. Clinical Symptoms: - Incubation: min - 3 hours - Duration: 3 hr / several days - Watery Diarrhoea, Nausea, Vomitting. - Burning Sensation in mouth (metallic taste) - Urticaria (rash) - Facial Flushing - Generalised Pruritus - Paresthesias |
|
|
What is Neurolytic Shellfish Poisoning:
|
- Associated with ingesion of contamined Shellfish (oysters, mussels, clams). NOTE: These outbreaks are associated with ingestion of Shellfish harvested in algal bloomd (Red and Brown Tides)
Pathogensis: Dianoflagellate alage: Gymnodinium Breve (recently renamed as Karenia Brevis). Produce Brevetoxin Clinical Symptoms: Incubation: <1-3 hours. Duration between 24-73 hours. Paresthesia, mouth numbness. |
|
|
What is Paralytic Shellfish Poisoning:
|
Pathogenesis:
- Dianoflagellate algae: Alexandrium species. Gymnodinium Catenatum, Pyrodinium Bahamenese, Gonyaulax Species, Produce Saxitoxins. Clinical Symptoms: Incubation - 15 min - 10 rs Duration: 3 days - Tingling and numbness of mouth spreading to extremities, GI symptoms are less common, ataxia (muscular in-coordination). Severe cases: muscle paralysis, respiratory paralysis. |
|
|
Describe NON-INFLAMMATORY DIARRHOEA:
|
- RESULTS FROM FOOD-BORN INFECTIONS
AETIOLOGICAL AGENTS: - Bacteria: E. Coli (ETEC:EPEC); V.Cholerae, Clostridum Perfringes, Bacillus Cereus (Diarrhoeal Form): Viral: Rotavirus; Noroviruses; Adenoviruses; Ither Viruses: NOT FOOD BORN INFECTION. Clostridum Difficile. Overview: Ingestion of organism present in the food. Production of toxins once intestines colonized. Symptomology: Acute Watery Diarrhoea without fever/dysentery: some patients may present with fever. Toxins: Enterotoxins for the bacteria. NO bacterial invasion of the intestines. |
|
|
Describe Escherichia Coli?
|
Biology:
- A member of normal intestinal flora - Member of Family of Enterobacteriaces - Gram -ve bacilli - Faculative Anaerobes - Considered major opportunistic pathogen. There are 6 different E. Coli associated with diarrhoeal disease. - Enterotoxigenic (ETEC) - Enteropathogenic (EPEC) - Enteroadhesive (EAEC) - Enterohemorrhagic (EHEC) - Enteroinvasive (EIEC) |
|
|
Describe the mechanism of :
- ETEC - EHEC - EAEC - EPEC - EIEC |
ETEC: Delivery of LT or ST enterotoxin into the cells
EHEC: intimate bacterial attachment causes actin condensation and microvillous efacement. The bacteria releases shiga toxin. EAEC: Delivery of Cytotoxin EPEC: - Adherence via BFP - Intimiate attachment - Actin Condensation and Microvillous Efacement EIEC: Invasion (engulfment), phagosomeal rupture, intracellular movment, lateral spread to adjacent cells. |
|
|
What is the # 1 cause of 'Traveller's Diarrhoea'
|
Enterotocigenix (ETEC) E.Coli
|
|
|
What is the Pathogenesis of Enterotoxigenic E.Coli (ETEC)
|
Pathogenesis: Following Ingestion of organisms, colonization of SI. With CFA's (Colonization Factor Antigen)
Once Colonized: They release 1 or 2 plasmids encoded ENTEROTOXINS Both are AB toxins: - Heat Labile (LT) Similar to Cholera Toxin, activated adenylate cyclase - Heat Stable (ST) Activated Guanylate Cyclase |
|
|
What are the Clinical Symptoms of Enterotoxins:
|
Clinical Symptoms:
- Incubation: 10h-3 days. Duration between 3-5 days. Self Limiting Infection. Rapid Onset of Diarrhoea, Nausea, Vomiting. |
|
|
What causes traveller's diarrhea in developing countries?
|
- Enteropathogenic E.Coli (EPEC)
|
|
|
What is the pathogenesis of Enteropathogenic E.Coli?
|
- Has a Plasmid-Born Bundle Forming Pilus (PFB) attaches to epithelial cells of Small Intestine.
- Once attached causes effacement of microvilli. - Leads to Osmotic Imbalance --> Watery Diarrhea. |
|
|
How do you manage Enteropathogenic (EPEC) ?
|
- Assess dehydration status and give Rehydration therapy.
|
|
|
What is Vibrio Cholerae?
|
Enviornment: Sea Water
Pathogenesis: AB Enterotoxin, A subunit activates adenylate cytclase. Similar action of LT toxin of ETEC. |
|
|
What it the clinical symptoms for Vibro Cholera?
|
- 2-3 days, duration up to 1 week
- RICE WATER STOOL Management: - 1 Replace Ionic LOSS. Oral and /or IV admin glucose solution with Normal Salt. Treatment: Tetracyclin |
|
|
in Cholera what agar do u use to differentiate it?
|
Thiosulphate-citrate-bile salts sucrose (TCBS) agar.
|
|
|
Clostridium Perferinges causes two types of enteric disease, they are?
|
1. Necrotic Enteritis (RARE)
2. Type A - Food Born Infection (a major cause of food-borne infections in the U.S.). |
|
|
1. Clostridume perferingies is ingested from what?
2. Igested of bactrium in what sort of meat? 3. What two toxins does it produce? 4. Clinical Symptoms? |
1. Meat or Gravy
2. Cooled Meat 3. CPE and B-Toxin. 4. 8-24 hr incubation. - Diarrhoea and Abdomina pain but: NO fever, nausea or vomiting. To Diagnose: presence of enterotoxin in feces. |
|
|
Describe 2 forms of gastroenteritis caused by Bacillus Cereus:
|
- Emetic and Diarrhoeal.
|
|
|
What is Bacillus Cereus known as what sort of syndrome and describe the LT and ST type?
|
- B. Cerus is chinese fried rice syndrome with incubation of 8-16 hours.
- LT ( activates adenylate cyclase) -> diarrheal form and is food born - ST (activates guanylate cyclase) -> Emetic form and is food poisoning. |
|
|
What is the #1 cause of viral diarrhea?
|
- Rotavirus.
- affects infants and children world wide. - 4 sereotypes: G1 - G4. G1 has a worldwide distribution, non group 1 (2 and 3) have limited destribution. G2 is mainly in china. Factors for incidence are: - Unsafe water - Inadequate sanitation |
|
|
What is the pathogenesis of ROTA virus?
|
- transmission is by fecal-oral route.
- (also water/air borne) - Incubation 1-3 days (after ingestin of cont. food) Duration is between 4-6 days. - Shedding may be 10 days - Histopathology shows, shortening and blunting of the villi. Patchy irregular intact mucosa. Mononuclear infiltrate of lamina propria. Diarrhoea results from the los of absorptive are and the flux of water/fluid across damaged surface. |
|
|
What are the clinical symptoms of rotavirus?
|
- Sudden onset of diarrhoea w/ w/out vomiting.
- Duration: up to 6 days (sometimes longer in immunocomramised). Complications: Dehydration which may be servere and life threatening. Managment: Rehydration therapy Preventation: Vaccination: Rotavirus Vaccine (Rotashield) |
|
|
How do you detect rotavirus?
|
in stool. (peak at 3 to 4 days of diarrhea).
Later agglutination Elisa Elcteron Microscopy. Electrophoresis. |
|
|
Describe the Norwalk Virus?
|
- Diarrhoea stool speciment from gastroenteritis patinets.
Epidimiology: - Estimated cause 50% of outbreaks of acute, non bacterial gastroenteritis( US) - Older children and adults ( Outbreaks in Camps, Schools, Nursing Homes) - Possibley the number 1 cause of GI tract infections in this age group of individuals. - Has winter seasonality. |
|
|
What is the pathogensis of Norwalk virus?
|
Transmission: prim. fecal-oral, water born and air born.
Virus multiplies in small intestine, produces transient lesions of intestinal mucosa. SPARES the large intestine (no faecal leukocytes). Shed in faeces. |
|
|
What are the clinical symptoms of norwalk?
|
Incubation: 1 to 2 days following ingestion. Duration of 1-2 days. Abdominal cramps, myalgias, malaise, headache, nausea and low grade fever and 1-2 days of diarrhoea.
|
|
|
Where do you get norwalk-like virus? transmission?
|
- Mostly restaurants
- Person-Person, Food-Borne, Water Borne. |
|
|
What are the two sereotypes of Adenovirus?
What is the pathogenesis: |
- 40 and 41
Pathogenesis: Infects: Epithelial Cells of the Pharync and Conjuctiva. Small Intestine and other organ systems. Virus replicates in the intestine and presents in stool. Causes watery diarrhoea with / or without vomitting. Associated with 5-15% cases of childhood diarrhoea (is the 2 to Rotavirus). |
|
|
What is Astrovirus?
|
- UK: Type 1
- Mexico: Type 2 Prevelant - Japan: Type 6 Cause 2-8% of sporaic cases in infants. (probobly 3 to Rotavirus). Infection throught year but peak in winter. |
|
|
With Toroviruses you have?
|
- Increased occurence of bloody diarrhoea and decreased occurance of vomitting.
|
|
|
How is Hep A spread?
|
- Fecal-Oral
- Person-to-Person - Poor Santation and overcrowding - Virus is shed in feces (10-15 days before the onset of symptms) |
|
|
Most Hep A infections are due to?
|
- 10% of all HAV outbreaks in US due to an infected food handler, ingestion of contaminated SHELLFISH.
|
|
|
Hep E virus, is prevelant in?
|
- Indian sub-continent
- Associated with sporadic outbreaks - Incubation: mean of 6 weeks |
|
|
Clostridum Difficile, is a major?
|
- It is a major nosocomial pathogen
- Causes a spectrum of intestinal diseases. (NOT associated with food-borne infection) - Uncomplicated antibiotic-associated diarrhoea --> antibiotic-associated colitis (psuedomembrane colitis) |
|
|
What is inflammatory Diarrhoea?
|
- Colonization and in most cases invasion of the intestines. Symtomology: Grossly blooy stool and fever may be present
|
|
|
Describe the Shigella Species:
What is the virulence factor for all Shigella? |
Group A to Group D.
- Closely related to E.Coli. - Enterotoxin that also act as neurotoxins. Cause meningitis, and coma, ulceration of the intestine. NAD Glycohydrolase: Destroys NAD in human cells whic shuts down metabolism resulting in cell death. |
|
|
What is Shigellosis?
|
- Term describing infection with Shigella Species.
- Most Severe: Shigella Dysenteria - Most Mild: Shig. Sonnei |
|
|
What are the clinical manifistations of Shigellosis?
|
- Water Diarrhoea/Abdominal pain and Vomitting.
- Mild / Moderate Dehydration - Dysentery: Water diarrhoea which progresses to small volume of bloody mucoid stool, with tenesums. |
|
|
What is the pathogenesis of Shigellosis?
|
- All are transitted via faecal-oral route particularly when sanitation breaks down. Invade Lower Intestine (at M cells or junction between cells)
Causes cell-death by apoptosis.` |
|
|
What is Bacillary Dysentery?
|
- Caused ONLY by Shigella Dysenteria Type 1:
Pathogenesis: - Shigela Toxin - Inhibis protein synthesis (inactivated 28S RNA). - Acts as enterotoxin: produces diarrhea, inhibits sugar and aminoa acid absorption in small intestine - Neurotoxin: affects the CNS (Do not know how). |
|
|
Children in day care can get ?
Where do you see Shigella Flexneri? |
- Shigella Sonnei (most common)
-- Also a minor cause of traveler's diarrhea. MNGMNT: Rehydration Therapy Antibiotic Treatment: Chloromphenicol, Ampicillin, Tetracycline is the most common. Diagnosis, Isolation and Identification: Stool, water and food Use MacConkey Agar (pale/colourless colonies) or S-S (Salmonella-Shigella agar). Shigella Flexneri - Sexuallya Active Gay Men. |
|
|
Describe Other Shigella Species:
|
- NO: lactose fermination OR citric acid utilization OR H2S production (except: S. Flexneri) OR gas from glucose fermination.
|
|
|
Describe the Pathogenesis of Salmonelosis Species:
|
For ALL FORMS:
Ingested Species reaches the GI tract -> Penetrate SI (sometimes LI) -> Multiply in M cells (w/in Peyre's patches)-> Released into the Lamina Propria. For Septecimia and Enteric Fever: -> Enter lymphatics and blood stream -> Multiply in Lymphoid Tissue -> Carried in blood to organs. For Enteric Fever: Infect Gall Bladder -> Replicate in bile-> Flow of bile re-infects SI * For Shingella, they do not get released into Lamina Propria but they get released into adjacent cells * |
|
|
What is Entercolotis (Gastrointeritis)
|
- Salmonella "Food-borne" infection
- Localized w/in SI - Causitive agent in UK Eggs (S. enteritidis). |
|
|
What is reptile associated Salmonellosis?
|
- Animal reservoir: lizards, snakes, turtiles.
- Infant/Children: Pets: direct/indirect contact Turtiles less then 4 in banned in US, 77% reduction in Salmonellosis. |
|
|
What is Enteric Fever?
|
Also known as Typhoid Fever
only caused by S.Typhi. In US seen in travelers to Asia, Mexico. Also Paratyphoid Fever: caused by S. Paratypho or/and S. Schottmulleri. |
|
|
What is the Asymptomatic Carriage in typhoid fever?
|
2-5% of typhoid patients become carriers. Excreting between 1-1,000 million S. Typhi/g faees.
Carrier State important in transmission. *Mary Molten* first carrier, she was jailed to prevent spread of typhoid. |
|
|
what is the Managment/Treatment of Typhoid Fever?
|
Rehydration Therapy
Antibiotics: Chloromphenicol/Ciprofloxacin. 3 vaccines. |
|
|
How do you Diagnose/Isolate and Identify Typhoid/Salmonellosis?
|
Isolate from stool, water and food.
Salmonalla-Shigella agar and/or MacCronkey agar. NO lactose fermentation; H2S production, Gas from glucose; Sereotyping. For S.Typhi: - History of travel, Rose colored spots on abdomen, Examination of blood (anaemia, leukoplakia, absence of eosinophils ). - Culture on S-S agar and +ve Widal reaction (agglutination of O and H antigens). |
|
|
What is the number 1 Food-Born disease in western society?
|
Campylobacterosis:
- unposturized milk, raw partially cooked poltry and contaminated water It is found in intestina tract of wie variety of wild and domestic animals (zoonotic), commericaly raised poultry, Normal commernsal of cows, Long-term commensal of sheep, Pigs and can be isolated from intestine of cats and dogs. |
|
|
What is the pathogenesis of Campylobacteriosis:
|
Once ingested, INVASION of Lower Intestine: Inflammatio and Bacterecemia suggest invasion.
Toxin Production: Endotoxin and Enterotoxin (ETEC LT-like water diarrhoea) and Verotoxin (Similar to Shigella Toxin Haemorrhagic Colitis) but the significance is not understood. |
|
|
What are the clinical Symptoms of Campylobacteria Species.
|
Incubation; 3-5 days. Duration between 2-10 days. Saymptoms: not distictive enough for easy recognition. Vomiting may be sligh, Diarrhea is often profuse (watery but may be bloody). Abdominal pain is often severe, Prostration is often severe; Pyrexia is often present. Differenced between Developing and Developed countries. (minor cause of Traveler's Diarrhoea)
MNGMNT: - Usually self-limited. Antibiotics are usually not given until ID of causitive agent. Erythromycin is given to eradicate C. jejuni from faeces. For severe abdominal pain: - Aminoglycoside, Chloraphenicol and doxycycline. |
|
|
What are the complication / association with the infection, Campylobacteira
|
- Reeactive Arthritis (in ~ 1% of the cases) Particulary knee joints, May last 6-12 months.
Acute Inflammatyro Demylenation Polyneuropathy { Gullian Barre'} Syndrome (30% of cases) AND acutre Motor Axonal Neuropathy. |
|
|
Enteroinvasive E. Coli Virus (EIEC) is similar to Shingella, except it?
|
Does not release Shingella Toxin, but pathogensis is similar by invasivness of enterocytes in lower intestine.
|
|
|
What is salmonellosis?
|
- Generalized Term used to describe infectin with Salmonella Species, 3 Types of Clinical manifestation:
- Gastroenterities: S Typhimurum, S Enteritidis, S. Newport causes traveler's diarhhea. - Septicemia/Bacteremia (focal infection) - Enteric (Typhoid) Fever. S. Typhi. |
|
|
Compare and contrast the three types os Salmonellosis?
|
1. Enterocolitis/Gastroenteritis:
Incubation 8 - 48 hrs Onset: Abrupt Duration: 1-4 days GI Symptoms: Nausea, Vomiting, Diarrhoea, at onset Blood Culture: -ve Stool Culture: +ve after onset. Septecimias: Incubation var Onset: Abrupt Duration: Rapid rise, spiking spetic temp. GI Symptoms: Often none Blood Culture: +ve during high fever Stool Culture: infrqeuntly +ve after onset. Enteric Fever: Incubation 7- 20 days Onset: Gradual, high plateau, typhoidal state Duration: Several weeks GI Symptoms: Early Constipation Blood Culture: +ve 1st-2nd week Stool Culture: +ve from 2nd week on |
|
|
What is the Pathological Mechanism of Salmonellosis?
|
FOR ALL FORMS OF SALMONELLOSIS:
- Ingested species reach the GI tract -> Penetrate SI (sometimes lower intestine) -> Multiply in M cells (w/ in the Peyer's Patches) -> Released into the Lamina Propria For Septicemia and Enteric Fever: - Enter Lymphatics and Blood Stream -> Multiply in lymphoid tissue -> Carried in blood to organs. For Enteric Fever: |
|
|
What is Yersinia Enterocolytica?
|
Rivals Salmonella - acute gastroenteritis (cooler climates).
Pathogenesis: Poorly understood. Invasive induced inflammatory response. Distal ilium (gut-associated lymphoid tissue). Adjacent tissue and mesenteric lymph nodes also infected (mimics appendicitis). Heat Stable Enterotoxin increases cGMP. Clinical Symptoms: 3-7 days incubation. Duration b/w 2-3 weeks. Self Limiting, abdominal pain and diarrheas. Mild fever but vomiting is rare. |
|
|
What is the Complication of Yersinia Enterocolytica:
|
- Post-Infective Reactive Arthratis (autoimmune arthratis). in small % of the cases. Pathogensis is poorly understood. MAYBE induces polyclonal T-cell stimulation (toxin). Nonspecific immune stimulation binding to B1 integrins on T lymphocytes.
|
|
|
What is the Diagnosis/Isolateion and Identification of Yersinia Enterocolytica
|
Stool sample considered late MacConkey or Specilizied Yersinia Media. Sereology: rising antibody titres in paired serum.
|
|
|
What is special about NON-CHOLERA Vibrio?
|
These Vibrio species are halophilic organisims. DO NOT agglutinate anti-O1 Sera.
|
|
|
How does Vibrio Parahaemolyticus causes?
|
- Ingestion of raw/poorly cooked seafood is the #1 cause of food borne infection in Japan
(SUSHI). Common in US (Shellfish) |
|
|
How do you treat V. Parahaemolyticus?
|
Tetracyclin (invasive so treat with antibiotics).
Pathogenesis: Reaches lamina propria, acute abdominal pain, vomiting and waery diarhhoea. |
|
|
What is Vibrio Vulnificus?
|
- Contaminated Sea water/ infection of cuts (Salt water abrasion). Virulent strain extensively invasive therefore requires tetracycline.
-> Fisherman |
|
|
What is Enteroaggregative (EAEC)
|
Origianally thought of as a sereotype of EPEC.
Mechanism: not folly understood, no EAF (enteric adherence factor) instead has AAF (aggragative adherence factor). Initial adherence to intetstinal mucosa and/or mucus layer by fimbriae. Strains produce mucus -> thick biofilm encrusted with EAEC. Cytotoxin production? causing damage to intestinal cells (sometimes results in bloody diarrhea). |
|
|
Describe Enterohaemorrhagic E. Coli (EHEC)
|
Also known as Verotoxin Producing (VTEC): Shiga Toxin Producing (STEC).
LIFE THREATENING CONDITION. |
|
|
What does EHEC cause?
|
1. Haemorrhagic Colitis (HC):
- bloody diarrhea that begins as abdominal cramps and watery - Incubation 3-8 days ater ingestion of contaminated food. mostly affects adults. ii. Haemolytic Uremic Syndrome (HUS): - 8-11% cases. Mainly affects children <5. Follow bloody diarrhea. Characteristic acute renal failure (increase in creatinine). Thrombocytopenia (redn in blood platelets) Microangiopathic Haemolytic Anemia (reduction in RBCs). iii. Thrombotic Thrombocytopenia Purpura: - Similar to HUS. Involves fever and neurological symptoms. |
|
|
What is the pathogenesis of EHEC?
|
Release of Cytotoxin known as Verotoxin. Biochemically similar to SHIGA toxin of S. dysenteria.
2 Types: Verotoxin 1 and 2 (VT1 and VT2). both AB toxins. Subunit A inhibits protein synthesis and causes HC. Also subunit A enters circulation, binds to renal glomerular endothelial cells causes HUS. MNG: Rehydration Therapy, antibiotics not recommended. |
|
|
How do you Diagnose E.Coli?
|
- E.coli are normal flora so it is difficult to distinguish pathogenic strain from non-pathogenic strain.
Methods: MacCorney Agar and Eosin Methyline Blue Agar (selective differential media). Serbitol MacConkey's Agar for EHEC. ELISA on toxin bound to antibody DNA probe to detect toxin genes or innaculate mouse adrenal cells: stimulation of adenylate cyclase by LT/ST for ETEC. |
|
|
What is a major nosocomial pathogen?
|
Clostridum Difficile. IT IS NOT associated with food-born infections.
C. difficile causes a spectrum of intestinal diseases ranging from uncomplicated antibiotic associated diarrhoea --> antibiotic-associated colitis (pseudomembranouse colitis). C difficile causes a spectrum of intestinal disease ranging from uncomplicated antibiotic associated diarrhoea to antibiotic-associated colitis (pseudomembranous colitis). Antibiotic associated Diarrhoea: minor concern but can be lifethretening. Antibiotics typically are: Ampicillin, Cephalosporins, Clindaymycin and Amoxicillin. Induced Pseudomembranous Colitis use of Antineoplastic agents (methotrexate) |
|
|
What is the pathogenesis of C. Difficile?
|
- INvoles only COLON. C.Difficile does not compete against the normal flora effectivly for attachment site. Antibiotic therapy desrupts normal flora and leaves attachment sites open.
Produces 2 toxins: Toxin A ( enterotoxin): leads to fluid accumulation in bowel (is also a weak cytotoxin) Toxin B (cytotoxin): decreases cellular protein synthesis and disrupts cellular microfillament system (similar to diptheria toxin) |
|
|
What are the clinical symptoms of C. Difficile ?
|
vary from mild diarrhoea to severe abdominal pain accompanied fever (>101F).
Diarrhoea: watery usually non-bloody (although 5-10% demonstrate bloody). Excess mucus and pus (or blood) Hypoalbumina and Leukocytosis are common. |
|
|
How do you diagnose C. Difficile?
|
- It is dificult to diagnose it, and distinguish it from ulcerative colitis and Crohn's disease.
Requires: Sigmoid scopic examination of colon (for presence of pseudomembrane) AND isolation of C.Difficile from stool of patients with diarrhoea associated with antibiotic therapy. |
|
|
How do you treat C. Difficile?
|
- Discontinou antibiotic use, symptoms should resolve in 1-14 days. If severe or no response treat w/ oral antibiotics. Vancomycin ("gold") standard or Metroniadazole (milder infections). Relapses have been shown in 15-20% of patients.
|
|
|
Describe the Causes of H. Pylori:
|
- Gram -ve / Urase +ve
Associated Causes: - Gastritis (stomach atrum) - Duedenal Ulcers - Stomach Cancer |
|
|
What is the epidimiology of H. Pylori?
|
- World Wide Destribution.
- Present in 20% in people <30, increases with age, reaches 50-60% age 60. Developing countries prevenlenace is higher. |
|
|
What is the pathogenesis of H.Pylori?
How do you Detect it? What about treatment/managment? |
- Gastric Colonisation is common BUT route of infection is unclear. Splits amonia from urea = alkaline enviornment.
Detection: Biopsy / Culture for present of H. Pylori. Treatment/Managment: Difficult, H.Pylori is sensitive in itro BUT DO NOT WORK monotherapy in vivo. 1st effective treament is: Triple Regiments: - Bismuth Salts, Metronidazole and Tetracycline, with good compliance (2-weels) = 90% cure Allternative: Acid-Supressive Drugs: Omeprazole (inactivates acid-secreting pump) will heal the ulcer but no cure for H.Pylori Alternative: Triple-Therapy: preserves protein pump inhibitor: Retains an imidazole (metroindiazole or trinidazole) Clarithromycin (macrolide), Omperzole, 1 week treatment = 95% cure. |
|
|
What is Chron's Disease?
|
- inflammatory bower Disease (IBD) and it is different from Ulcerative Colitis.
Chron's affects full GI tract, mouth to anus. Ulcerative Colitis affect the Colon only. The cause in contreversial. May be microbial. Ingestion of undercooked mean containing M. paratuberculosis. |
|
|
What are the Clinical Symptoms of Crohn's Disease?
|
- Fever, Abdominal Pain (lower right areas), severe anorectal complication of fistulas, fisues anre peri-rectal abcesses. Diarrhoea, Fatigue, Due to an immune response?
The goals to therapy is to control inflammation, correct nutriontal deficiences and relive abdominal pain. Diagnosis: Look for tiny longitutdinal fissues or ulcers (giving a cobbelstone apperence). Culture: Herrold's egg yolk and mycobactin: 12-16 weeks incubation. Unrelible (it is not routinly performed). PCR: Colon Biopsies. |
|
|
What are the Gastrointestinal Abscesses?
|
- Peritonitis Appendicitis, Diverticulitis, Intra-Abdominal Abscess, Liver Abscesses, Pancreas Absecess.
NB: Healthy GI tract has > 300 bacterial species. Normal Specific and non-specific immune response prevents spread to extra-intestinal sites but occasionally compromised allowing invasion. |
|
|
What is the pathogensis of Gastrointestinal Abscesses?
|
Pathogenesis:
Compression occurs when: - Reduced O2 tension and oxidation-reduced potential. - Impaired blood supply: necrosis of tissue, growth of facultative anaerobes. iii. Association: vascular disease, trauma, surgery, presence of foreign bodies, malignancy, radiation therapy, injection of vasoconstrictive agents, shock, cold or edema. Why? they all provide anerobic envionment, impaired host defences which enables these opportunists to grow. |
|
|
What are the clinical symptoms of Gastrointestinal Abscess?
|
- Peritonitis: pain abdominal distention, diffuse muscle spasm, tenderness and rebound tenderness decrease/absent paristalasis, rigidity o abdomnal wall, tenderenss on rectal or vaginal exam, fever and leukocytosis.
Appendicitic/Diverticulosis: Abdominal Pain, Peritoneal Irritation, Fever and Leukocytosis. Pylephlebitis and Liver Abscess: Chills, Fever, epigastric or right upper quadrant pain, nausea, vomiting, enlargment and tendereness of the liver. Pelvic Abscess: pain, deep denderness in 1 or both lower quadrants, fever, urinary frequency, dysuria and diarrhoea with passage of mucus in the first stools. Rectal or Vaginal examination may reveal tendrenss. Pancreatic Abscess: acute, severe epigastric pain. Recent history of excessive ingestion of food and alcohol. Nausea and vomiting are common. ` |
|
|
What is the treatment options for Gastrointestinal Abscess?
|
- improve vascular perfusion (correct fluid and elecrolytes) Combat effects of bacteria and toxic metabolites, reduce paralytic ileus, eliminate primary source of infection, aspirate infected exudate (drain primary lesion), treat local or distant complications.
Antimicrobial Therapy: Anaerobes are resistant to penicillins , cephlosporin and most amino glycasides. Possible: Cloramphenicol (succinate), Bacterioide Fragilis, Metranidazole, Gentamycin, Tobramcin and Amikacin. |
|
|
How do you manage Diarrhoeal Disease?
|
Oral Rehydration: Giving till normal rehydration is restored. Determined by: Clinical Conditions/Body weight. Sodium: 150 155 mm/L. Glucose 200-220 mmol/L. Potassium: 2-5 mmol/L. THIS SHOULD BE THE FIRST LINE OF TREATMENT:
Intravenous Rehydration: Shock, Exhaustation precluding oral feeding and oral rehydration failure. Antiemetic Drugs: Reduce Fluid Loss: Oral Rehydration is Effective. Antidiarrhoeal drugs: Are rarely successful they reduce gut motility and allow accumulation of fluid faces. |
|
|
Zonnosis: Usually Human infection is dead end, but there are some human-human transmisssions..
How can infection be prevented? |
Safe food production: healthy flocks and herds, avoid sewage to fertalize crops.
Food Manufacture process: hygienic slaughter and meat packing, rodent free storage of crops, store at chill or refrigirated temp. Hygenic packaging, cold chain during distribution. Domestric and Commercial Food Hygiene: Adequate refrigeration, acoidance of cross-contamination, usage within spoilage dates, adequate deconamination of food by washing and/or cooking, personal and kitchen hygiene. |
|
|
What are the human-human infections?
|
- Campylobacter and Yersinia infection: via feta-oral route .
- Pneumonia Plague: respiratory droplets - Relapsing Fever: Body Louse - Salmonellosis: Fecal Contamination of food. |
|
|
What are the predisposing factors to zoonosis?
|
Occupational exposure: vets, famrers, abattoir workers, zoo staff, pet shop staff, pet owners.
- Leisure exposure: camping, outdoor activities (hiking, hunting, fishing, swimming) - Ingestion of contaminated food. |
|
|
What are the routs of aquiring a zoonotic infection?
|
- Direct Skin Penetration
- Inhalation - Ingestion - Arthropod Vector ( Ticks, Fleas and Mites). - Animal bite or scratch |
|
|
Most zoonotic infection are transmitted by one route, exceptions are:
|
- Taularemia (F. tularenis): direct skin penetration, inhalaton, arthropod vector
- Anthrax (B. Anthracis) - direct skin penetration, inalation - Plague (Y.pestis): inhilation, arthropod vectors. |
|
|
Which diseases fall under Catagory "A" of warfare?
Which ones are "B" ? |
- Bacillus Anthracis - Anthrax
- Clostridium Botulinium Toxin: Plague - Variola Major: Small Pox - Francisiella Tularenisis - Tularemia - Viral Hemorrhagic Fevers: Ebola, Marburg, etc. B: Brucella Species - Brucellosis Clostridum Perfiringes - Epsilon Toxin - Burkholderia Mallei - Glanders - Coxiella Brunetti - Q Fever - Staph Aureus - Enterotoxin B. |
|
|
Decsrobe monkeypox: Orthopoxovirus.
|
- linar DS DNA
- Similar to small pox, milder but also includes LYMPHODENOPATHY - Papular rash that progrsses through vesicles > papules > umbilication > encrustation. - Linked to Gambian Giant Rats, transmited to Prairie dogs which were sold as pets. |
|
|
Decribe the Plague, Yersinia Pestisi
|
Important Reservoirs: small burrowing animals and rodents (gerbils, voles, ground squirrels, voles), urban rat.
- Lymphohematogenous Infection - Often fatal to the individual animal but it establishes itself in a meta-stable balance within a host population. (Dynamically maintained in rodent population for extended periods). Current Reservoirs: - Semi-Arid regions in the southwestern US. - Southeast Asia and Graddlands of Central Asia - Not Found in: Western Europe, Africa or Australia. Vectors: Fleas (animal-animal and human-animal transmission). > 80 species of fleas world-wide proven as vectors. - Flea bites bacteremic mamals. - Bacilli multiply in insect midgut (form biofilm in the proventricular [valve-like organ], blocking normal feeding, regurgitation at subsequent meal into the bite wound. |
|
|
Describe the various forms of human plagues [Yersinia Pestitsi]: - it is a gram negative bacteira looks like a safety pin
|
Bubonic:
- Infection of draining lymph nodes in vicinity of flea bite. - "Buboe" - painful swelling, can occur in inguinal, axillary or cervical lymph nodes. Septicimic: - Can be primary or as a result of bubonic or pneumonic plague. - Almost always fatal - Purpura in skin - "black death" Pneumonic: - Can be primary or as a result of bubonic or septicemic form. - Can also be acquired and transmitted via infected droplets (this form of disease is extremely contagious). - Severe bronchopneumonia |
|
|
What is the pathogenesis of Yersinia Pestis?
+ Where do you detect the specific forms of the plague? |
Virulance Factors:
- F1 protein capsular antigen - Yops [Yersinia outer protei] -- Affects phagocytic Response - Liopooligosaccharide (LOS) - Yersiniabactin (siderophore) - Skin -> Lymph Nodes -> most are killed by PMNs. - Some are taken up by tissue macrophages, and replicated. -- "adjustment phase", upregulation of virulence factors relevant to extracellular survival. - Subsequent escape from macrophages and replication -> Systemic Infections. Appropriate Virulance Factors will be necessary to enable different stage in the lifesycle: 1. Maintenance of reservoir in rodents. 2. Infection of accidental hosts, e.g. humans. Confirmaion dependent on detection of bacilli in appropriate speciment: - Blood for septicemic form - Sputum for pneumonic form - Buboes or local skin lesions for bubonic form. Culture hazardous: PCR safer and mroe rapid (using primers based on F1 Gene Sequence.) |
|
|
Factor: Recreational Activities.
Describe Leptospirrosis: Leptospira Interrogans. |
Synonyms: Mud Fever, Swamp Fever, Sewerman's Flu.
- First Phase: flu-like symptoms (fever, vomiting, nausea, headache, abdominal pain and muscle aches. - Second Phase: aseptic meningitis or generalised illness. Most Severe form (Weil's Disease): Altered liver and kidney function, circulatory collapse. CNS can also become involved, haemorrhagic rash. Transmission: Contaminated Water Visualized by dark field microscopy. Capable of extended survival in water and warm moist soil. Bacterium causes disease in various wild and domestic animals (included: rodents, cattle and dogs). Pathogenesis: - Breaches in skin barrier allow entry. Can also gain access via conjuctiva and via mucosa of GIT following ingestion. Vasculitis that causes small blood vessel endothelial damage is probobly a contributing factor. Diganosis: - Serology [Microscopic Agglutination Test(MAT)] - Body Fluids can have low numbers of spirochetes, therefore darkfield micrscopy is NOT recommended. - Culture Difficult and requires specialized medium. |
|
|
Factor: Expansion into the rural areas
Describe: Ebola and Marburg. |
Filoviridae: ss negative sense helical RNA
- Filamentous - Highly Pleomorphic (filo: latin for thread). - Can cause lethal haemorrhagic disease. - Mortality rate is 30-80%. Symptoms: - Being w/ severe headache and malaise - High Fever - Haemorrhagic Manifestations after 5-7 days. - In severe cases, bleeding from multiple sites (eyes, gums, body orfices). Reservoirs: small mamals, chimpanzees, bats. (Marburg because it was named after where the outbreak happened). Ebola virus is antigenically different. Persno - Person infection can occur. Pathogensis: Not well understood, Role of viral membrane glycoprotein is suggested for some Ebola subtype. Virus replicates in liver and most body tissues. Death is due to: Elevated Vascular leakage: Releases fluid and blood into extravascular spaces. Damage to blood vessels and spontaneous bleeding probably associated with platelet dysfunction and endothelial damage. NO antivirals availbale. Extensive supportive treatment is necessary. |
|
|
Describe the Encephaliis: Nipah Virus.
|
Newly emerged Paramyxovirus:
Reservoir: Fruit Bats --- Pigs infected and developed respiratory illness. --- Clearance of land for new iairport --> bat migration to proximity of farms. |
|
|
Describe the different stages of the Lyme Disease.
|
They are helical rods (spirochetes) associated with a progressive, multisystem infection.
Stage 1: localised skin infectioion: Erythema Chronicum Migrans (spreading annular rash), Malaise, Headache, Fatigue, Rigors, and Neck Stiffness. Stage II: Disseminated Infection, Weeks to Months, Heart or Nervous System Abnormalities: Musculoskeletal system is affected: Joint Abnormalities. Stage III: Persistent Infection, Chronic Skin and CNS or Joint Abnormalities. |
|
|
Describe the Epid. of Lyme Disease?
|
- 1st Identified as infectious conditions in 1975 following a cases in Lyme, Connecticut.
- Now most common reported arthropod-borne illness in the USA and Europe. - Cases also reported in China, Japan and Australia. Transmission occurs by Ixodes Species (Blacklegged/Deer) ticks that have become infected following feeding on infected animals. Natural Hosts: Wild and Domesticated Animals: mice, deer, sheep, cattle, horses and dogs. Occurances increase in June and July. |
|
|
What is the pathogensis of Lyme Disease?
|
- Binds to integrins, proteyglycans or glycoproteins on host cells or tissue matrices.
- Able to bind plasminogen and its activators on the bacterial surface. - Fibrinogen receptor binding by outer-surface protein: beneficial for establishing infection and disseminating via blood vessels. - A protein called Bgp (borrelia Glycosaminoglycan-binding protein) attached to sdie chains of heparan sulfate (endothelial cells), and to both heparan sulfate and dermatan sulfate (on neuronal cells). - Two Decorin-Binding Proteins (DbpA and DbpB) bind decorin. Might account for the finding of spirochetes in associate with collagen in extracellular matrix of the heart, nervous system and joints. Immune Response evasion: - Able to antigentically vary a surface lipoportein. - Spirochete surface protein bind certain complmeent inactivators -> inactivation of C3b: protection against complement. |
|
|
How do you diagnose Lyme Disease
|
Diffucult and time required to culture the spirochete mean that diagnosis is usually made serologically.
ELISA most widely used. Infection with other spirochetes may result in cross-reacting antibodies (e.g. patients who have had Lyme disease may give a positive FTA-Abs [for T.Palladium)] Controlling the disease: - Education - Use of tick repellents and proper clothing in endemic areas. |
|
|
Factor: Consumption of animal products and farming practices:
1. Describe Haemorrhagic Colitis: E. Coli O157:7 2. What is vCDJ |
1. Hemorrhagic Colits caused by E.Coli.
vCJD: Prions, caused by "prions" into the food chain wat due to recycling of anmal carcasses into protein feed that then fed into other animals. Cause transmissible spongiform encephalopathy (TSE_ - Creutzfeld-Jakob Disease (CDJ) Consequence association is made with prions and a virulent variant (v) CJD infection in humans. Features of vCDH compare to CJD: - shorted duration - Shorted incuation period - More rapid onset and progression of symptoms - Younger patient age group affected. |
|
|
Describe Anthrax: Bixxulus Antharcis
Factor: Contact with animal/products. |
Anthrax: Bacillus Anthracis
Gram +ve Endospore formers Animal Infection (mainly herbivores) is via acquisition of Bacillus endospores from their pasture land. Manifstation of human anthrax: 1. Curaneous: ulcarative collitis -- Lesions progresses from "inset-bite like to erythematous papule, through vesicular and ulcerative stages. -- "Anthracis" -- "coal like" = refers to black eschar. 2. Pneumonia: -- Spore inhalation of subsequent germination causes non-specific respiratory symptoms (mild-fever, non productive cough, malaise). -- Progresses to respiratory distress and cyanosis. --- Bacterium disseminated into bloodstream and CNS. |
|
|
What is the pathogensis of anthrax?
|
Major Virulence components:
1. Antiphagocytic poly-gamma-D-glutamic acid capsule -- 1 Serotype -- Plasmid encoded: pXO2 2. Production of a potent exotoxin with multiple activites: Plasmid Encoed: pXO1 -- Protective Antigen (PA) ----- Facilitates cell binding and uptake into cell -- Lethal Factor (LF) ----- Inhibits activity of Mitogen-activated protein kinase kinases (MAPKK) -------- disruption of signaling and normal cell functions. -- Edema Factor (E) ---- Adenyl Cyclase ---- Binds with calmodulin (eukaryotic Ca2+ binding protein) and this complex elevates host cell cAMP levels. Fully Virulent Levels have pXO2 and pXO1. Look at page 52-54/12 for details |
|
|
What is the toxcity of Anthrax?
|
PA binds to cellular receptors: it is cleaved into 2 proteins (20kD and 60kD) -> forms a hepamer (prepore) -> endocytosis and acidification -> pH 5.5 (prepore forms in endosome membrane) -> LF and EF translocation into cytosol.
|
|
|
Describe Brucellosis: Brucella Species.
|
Chronic illenss:
- Feer (39.4 - 40C), late-afternoon/evening sweats, weight loss. - Weeks -> months duration - Cycling night Fevers - Weight Loss Genitourinary infaction of various farm animals (sheep, cattle) Exposure can be direct contact, inhlaition or through ingestion of contaminated products, e.g. unpasteurised milk, goats cheese. Endemic in Middle East and Latin America: Microbial Characteristics: Gram Neg, Rod Non- Endospore Former Specific species involved in himan infection varies with anmial reservoir: - B.Suis - PIGS - B. Melitensis - Sheep and Goats - B. Abortus - Cattle Exposure. Various Routes. |
|
|
What is the pathogenesis of Brucellosis:
|
- Pathogenesis:
-- Faculative intracellular parasites: epithelial cells and professional phagocytes. -- Multiply in macrophages of R.E.S --- Intracellular surviral mechnaisms include: ------ Myloperoxidase system supression ------ Inhibition of phagosome-lysosome fusion ------ impairment of cytokine production by monocytes. In absence of control of infection: formation of granulomas and localised bacterial replication - However in <25% of cases have detectable enlargment of RE organs. When enlargment observed, splenomegaly is most cmmon finding, Lymphodenopathy and hepatomegaly can also occur. Subsequent release of bacteria into circulation -> recurrent chills and fever. Diagnosis: - Due to variability of clinical presentation, lab confirmation is essential. Isolation of Brucell from blood or appropriate biopsy speciment. -- Slow Growing. Can also diagnose serioloigically: -- Agglutination tests ( Rose Bengal, Wright's tube, Wright's Card and Wright-Coomb) -- Indirect immunofluorescence -- Complement Fixation -- Coenzyme-linked immunosorbent assays. PCR assays highly sensitive and specific but often unavalible in endemic/resource-poor areas. Prevention: - Minimize occupaton exposure. - Reduce food contamination through pasteurisation. - Animal Immunisation (no human vaccine) and culling. |
|
|
Describe the Avian Influenze "Bird Flu": Influenzavirus
|
"bird flu" = influenza virus type A infection of birds
Can cause mild or severe forms of diease in birds. 16 Haemagglutinin (HA) and 9 neuroaminadase (NA) types: all found in wild waterbirds: Four Known Strains to cause human disease: H5N1***, H7N3, H7N7, H9N2 * H5N1 has jumped to humans before: a. Hong Kong 1997 b. Hong King in 2003 -> Co-Infection and genomic recombination: low virulent viruses become highly pathogenic via mutation after circulation in a population. In Humans Infection with H5N1: - Unusual Severity - Rapid Progression - High Fatality - Symptoms indicating lower RT involvement occur early in disease, almost all patients developed pneumonia. |
|
|
Describe Francisella Tularensis?
|
Transmission: Direct Contact (bite of infeted animal), insect bite, contaminated food or water, aerosoles.
Charachtaristic bipolar staining using 10% carbol fuchsin. 2 biovars: - Jellison Type A and B. A- Prediminantly in North America, tick transission. B- Europe,Asia,North America, Mosquito transmitted. Carbohydrate capsule Known to be capable of intracellular survival, e.g. macrophage. Very low infectious dose: 1-10 bacilli Initial Symptoms: - fever, headache, rigor, malaise. Subsequent symptoms associated with disease reflect entery route of infection: - Ulceroglandular form: Tick bite (most common). - Typhoidal Tularaemia: contaminated food/water. -- "Typhoidal form" - similar presentation to typhoid fever. - Pneumonic Tularaemia: Aerosols -- Pneumonic Form, or more generalizes, similar to the typhoidal form. - Oculoglandular form: conjuctival inoculation -- Painul, purulent conjuctivits. From initial infection, spreads to RES forming granulomas. Infection can follow chronic and relapsing course. Dangerous to handle: Cat 3 continment necessary. Requires special culture medium. Glucose-Cystein or cystine heat agar usually diagnosed serologically. |
|
|
Describe Coxiella Burnetti, Q fever.
|
Causes Q Fever:
Reservoir: wild and domestic ungulates including cattle, goats, rabbits and also cats and dogs. Transmission routes; - Inhalation - Direct Contact ( contaminated milk, animal urine, feces) Symptoms: - Majority of infetions are sub-clinical - Non-specific febrile illness, fever, chills and headache. Mild dry hacking cough sometimes present. Chronic Infection uncommon, can take the form of : - Granulomas - Endocarditis - Meningoencephalitis Obligatey Intracellular Bacterium Inhalation -> Entry into alveolar macrophages. - Highly infectious Diagnosis is a specific antibody demonstration: Complement Fixation Test or indirect IFA. |
|
|
Describe: West Nile Fever:
|
- ss(+) RNA
- Factors: travel and transport/shipping. Neuropathogen of birds, horses and humans. Found in: Asia, Africa, Europe, and Middle East. Has spread rapidl across the US> Spread linked to miagration of infected birds. Mosquito Born. Clinical Presentation: - 80% of infected individuals are asymptomatic - 20% develop mild flu-like symptoms - <1% develops encephalitis, axonal polyneuropathy. Which ways did the mosquito enter/have been introduced to US: - Legally or illegally imported plans and flowers, where the mosquito, larve or eggs were present. - Infected humans travelling from endemic areas - Mosquitos carried via planes or as larvae on transatlantic cargo ships. - Migratory birds. |
|
|
Describe Hantavirus Pulmonary Syndrome: Sn Nombre Virus
|
Factor: Climate Change
- Severe Pulmonary Syndreom in Arizon, New Mexico, Colorado, Utah. High Mortality: 20-50% Symptoms: Fever and vascular leakage -> pulmonary edema followed by shock. Contributing Factors: - Unusually mild autumn -> increased harvest of pinenuts -> Explosion in population of deer mice. - Human infections then occurred following due to contact with contaminated mouse urine or droppings. Bunyaviridae Family: - Enveloped - 3 Segmented, single stranded (-) RNA - Most common hantavirus involved: Sin Nombre Virus (SNV) - Hantaviral Infections: -- Hemorrhagic Fever with renal syndrome (HFRS) - Eurasia -- Hantavirus Pulmonary Syndrome (HPS) - Americas Mechanisms of pathogenesis is poorly defined. Immunopathoglogy suggested as key contributer. |
|
|
Describe the Different Rickettsial Infections (Contributing Factor: Climate Change)
|
Rockey Mounted Spotted Fever: Rickettsia Rickettsi, Vector is Tick, Reservoir: Rodents, Dogs
Mediterranean Spotted Fevers: V: Rickettsia Conorii, Tick, R: Rodents and Dogs. Epidemic Typhus: Rickettsia Prowazekki, V: Body Louse, R: Humans Murine/Endemic Typhus: Rickettsia Typhi, V: Flea, R: Rodents. |
|
|
What are the characteristis of the Rickettsiae?
|
- Obligate intracellular parasite
-- Replicate in cell cytoplasm Small: 0.3 - 0.5 um - 1um Human Pathogens usually present in endothelial cells. Typically Gram Negative Cell Wall -- Stain poorly with gram stain: Giemsa better for visualisation. Rickettsia Divisible into two groups based on antigenic proporties. 1. Typhus Group -- R. Prowazekki and R. Typhi. -- Transmission is via contact with insect feces. Spotted Fever Group: -- 9 Different Rickettsial Species -- Mostly Transmitted via tick bites. -- Range of syndromes: Rickettsialpox, African Tickbite Fever Cat Flea Typhus. |
|
|
Describe, Epidemic Typhus: Ricketssia Prowazeckii.
|
Transmission: Pediculus Body Louse.
Symptoms: - Characteristic Ricketssial Headache - Steady high dever - Exanthem: maculopapular. Appears first on trunk and spreads centripedally to extermities. (*opposite pattern to that of RMSF) - Complications: sometimes fatal -- Mycarditis, Vascular Collapse -- CNS dysfunction |
|
|
Describe Endemic Typhus: Rickettsia Typhi:
|
- Tranmission = rat flear
- Illness beings with headache, myalgia and fever. - maculopapular rash. Similar spread to typhus. |
|
|
Describe Rocky Mountain Spotted Fever:
|
- Symptoms: Fever, headache, toxcitiy, mental confusion, myalgia,
Characteristic Rash: appears on 2nd or 3rd days. -- Small Erythematous Macules that quickly become perechial. -- Rash appears on writs or ankles and spreads inwards to the trunk within hours. ---- Extends to cover palms of the hands and soles of the feet. Transmission - ticks In USA, types of tick responsible varies with georgraphic area: - Wood Tick - Western USA - Dog Tick - Eastern USA - Lone Star Tick - Southwestern and Midwestern USA Untreated Cases can develop fatal complciations: - Disseminated intravascular coagulation - Thrombocytopenia, Encephalitis, Vascular Collapse - Renal and/or heart failure 2/3rd of cases of RMSF are in children <15 years old. |
|
|
How do you diagnose Rickettsial Infections?
|
Diagnosis and Initiation of treatment is based on history, clinical presentation signs and symptoms, presence of endemic region, etc.
Can subsequently diagnose serologically: antibodies apparent after 6-7 days of illness. -- For RMSF: indirect immunofluorescence assay considered the reference standard PCR also available. |
|
|
What is an Arboviruses?
|
Arbo: Arthropod Borne
- Large group of viruses, variety of different genra involved. - Infection results in one (or several) disease presentation. Can be either: - Inapparent Infection - Acute Encephalitis - Infection of the visceral organs - Febrile Illness |
|
|
What are the three types of arbovirus transmissions?
|
1. Urban: (i.e. Denuge)
- HUMANS -> MOSQUITO - > HUMANS - MOSQUITO - > HUMANS. Sylvatic (i.e. Yellow Fever) sometimes multiple reservoir can be involved. Mosquito - > Nonhuman Verterbrate Reservoir -> Mosquito - > Nonhuman Vertebrate Reservoir. (from the mosquito, it can be transmitted to humans). Arthropod Sustained: - Arthropod transmits microorganism vertically, cycle of infection can be amplified by spread to and from small mammals. |
|
|
What is the pathogenesis of arboviral infection?
|
1. Insect Bites -> Virema (Virus replicated in RES and Vascular Endothelium) -> Virus localized in target organs -> Cell Necrosis and Inflammation.
|
|
|
How do you diagnose Arbovirus infection?
|
1. Serelogy, e.g. ELISA, Indirect Immunoflouresence
2. Viral Isolation -- Time-Consuming 3. Detection of specific RNA using RT-PCR. |
|
|
Describe Dengue Haemorrhagic Fever/Shock Syndrome:
|
- ss+RNA
- Enveloped - 40-70 nm diameter. Four Related Virus Serotypes (1-4) -- Usual Presentation: acute febrile illness --> Chills, headache, retro-orbital pain, aches ("break-bones fever") --> Changes in small dermal blood vessels observed -> Maculopappular rash. More severe form: haemorrhgic fever (DHF) ~ 5,000 cases per year: mostly in children - Ocasionally accomppanied by shock (Denuge Shock Syndrome) Both DHF and DSS are usually observed following a subsequent infection with a different serotype. - Role of immunopathogenesis: enhancement of infectivity. |
|
|
Describe Yellow Fever: and yellow fever virus:
|
- Flavavirus:
ss + RNA Enveloped 50-70nm diameter. Georgraphic Distribution: Caribbean and Central America, South America (Amazon Valley) and Central Africa. from Atlantic coast to Sudan end Ethiopia. Abrupt onset of fever, chills, headache and haemorrhage. |
|
|
Two Steps of Emergence?
|
- Introduction (many are zoonotic)
- Dissemination |
|
|
What stops some pathogens from spreading from animal-human?
|
- Species Barriers
-- Receptor Specificy -- Co-Receptors -- Host Specific Transciption Factors -- Innate Immunity |
|
|
What is the typical age for death by pneumonia?
|
- Elderly and Infants
|
|
|
What is a public health response to Influenza?
|
- Srveillance and Early Warning
- Immunization - Antiviral Agents for prophylaxis or therapy - Other non-pharmaceutical interventions - Public Education |
|
|
What is the emergence of influenza?
|
- novel "new genes" are usually avian
- May pass thru intermediate hosts to reassort (e.g. pigs) - 1918 ("Spanish") Influenza is worst known to man. |
|
|
What is the emerging infections two-step?
|
Opportunities Increasing For both Steps:
- Changes in Land Use - Rural to Urban Miagration - Internal Displacement - Globalization of people and goods, travel, international migration. - Medical Technologies |
|
|
SARS Coronavirus : What is the Natural Host?
|
- Probably Bats
- Civet Became Incidentally Infected -- Link to Human Food: food animal - Seropositives in other mammales tests (raccoon, dog, badger) |
|
|
How does virulance evolve?
|
Pathogens and their hosts may co-evolve
Many Possible Outcomes: - A pathogen may become specialized to a host (smallpox, many human herpesviruses) - On first contact, pathogen may be highly lethal, initally killing most susceptible infected hosts (myxoma virus and rabbits in Australia) |
|
|
What limits pathogen virulance?
|
- Host Response or susceptibility may be a factor
- Pathogen may run out of susceptible hosts, so only resistant individuals reamina. Examples: - Myoxoma in Australia (Rabbits) - Possible Sicke Cell and Maleria - Possibly Cholera and Cystic Fibrosis . |
|
|
Define Bioterrorism?
|
- Bioterrorism: intentional or threatened use of viruses, bacteria and fungi, or toxin from living organisms to produce death or disease in humans, animals or plants.
|
|
|
What is the emerging infections two-step?
|
Opportunities Increasing For both Steps:
- Changes in Land Use - Rural to Urban Miagration - Internal Displacement - Globalization of people and goods, travel, international migration. - Medical Technologies |
|
|
SARS Coronavirus : What is the Natural Host?
|
- Probably Bats
- Civet Became Incidentally Infected -- Link to Human Food: food animal - Seropositives in other mammales tests (raccoon, dog, badger) |
|
|
How does virulance evolve?
|
Pathogens and their hosts may co-evolve
Many Possible Outcomes: - A pathogen may become specialized to a host (smallpox, many human herpesviruses) - On first contact, pathogen may be highly lethal, initally killing most susceptible infected hosts (myxoma virus and rabbits in Australia) |
|
|
What limits pathogen virulance?
|
- Host Response or susceptibility may be a factor
- Pathogen may run out of susceptible hosts, so only resistant individuals reamina. Examples: - Myoxoma in Australia (Rabbits) - Possible Sicke Cell and Maleria - Possibly Cholera and Cystic Fibrosis . |
|
|
Define Bioterrorism?
|
- Bioterrorism: intentional or threatened use of viruses, bacteria and fungi, or toxin from living organisms to produce death or disease in humans, animals or plants.
|
|
|
What are the types of surveillance systems .
|
Passive: Initiated by provider
Active: Initiated by the health department Sentinel: Designated facilities/vulnerable populations |
|
|
Reporting System is:
|
ProMed Mail
|
|
|
A Layered Defense Required:
|
Research:
- Ecology of Infectious Disease - Pathogenesis of Infectious Diseases - Understanding the barriers and enables for successful cross-species transmission, - Better understanding of the biological basis of transmitability Lab Capaciy: - For Diagnosis Public Health - Preparedness - Surveillance - Public Health Response |
|
|
What is the difference between BIOLOGICAL and CHEMICAL weapons?
|
BIOLOGICAL: Organic or Inorganic compounds which have a toxic effect on living things.
CHEMICAL: Living Organisms and/or Viruses. |
|
|
Who are the first responders for:
1. Nuclear Weapons 2. Chemical Weapons 3. Biological Weapons |
1. Civil Authorities
2. Civil Authorities and EMT 3. Hospital Staff (Effects are Delayed) |
|
|
Describe the BioSafety Levels (1-4):
|
BLS 1: agents not know to cause disease in healthy humans
BLS 2: moderate potnetial hazard -> BLOODBORNE PATHOGENS BLS 3: May cause serious lethal disease by inhalation --> AIRBONE PATHOGENS BLS 4: highly transmissible airborn disease -> need maximum protection |
|
|
How do you Weaponize the Natural Infectious Agent
|
1. Grow - the infectious agent to a very large volumes and high concentrations without loss of virulence .
2. Enhance (or maintain) the virulance 3. Stabilize - the infectious agent so that it may be transported and stored w/out loss of virulence. 4. Interface the infectious agent with the delivery system. |
|
|
How do bioloical weapons get disperesed?
|
- Must release at night
- Must release as dust or neubulized liquid spray if aerosolized is the goal. - May become difficult to remain clandestine - Cannon use explosive dispersal - Alternatively, must release as containment of food or water. |

