![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
A Few Terms hnotes |
•Pathogen - organism that produces disease •Opportunistic Pathogen - infects host with weakened immune system (compromised) •Carrier - infected individual, potential source of infection (no symptonms tho) •zoonoses: diseases transmitted from animals to humans •vectors: organisms that can transmit diseases to humans (ex: mosquitoes, ticks, fleas) |
•Pathogenicity –ability to produce disease •Virulence– degree of pathogenicity •Virulence factors or determinants: genetic, biochemical, structural features -contribute to virulence •latency: pathogen stops reproducing, becomes dormant, can become active again later. •pathogenicity islands: region of DNA, large segments of chromosomal or plasmid DNA, that encodes virulence determinants. Absent in nonpathogenic strains •G+C content: content different from the rest of the bacterial genome. |
|
|
How do we measure virulence? hpic |
•Measure the infectious Dose 50 (ID 50) -# of pathogens required to cause clinical disease in 50% of the inoculated hosts. •Lethal Dose (LD 50) -# of pathogens required to kill 50% of the hosts. |
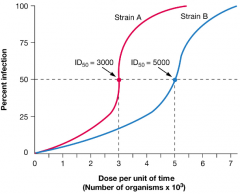
|
|
|
Viral replication cycle |
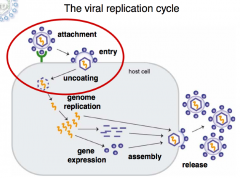
|
|
|
|
Viral Attachment |
- fill in later |
|
|
|
Viral Spread |
•Viruses can spread by using blood, neuronal and lymphatic systems •Syncytia: multinucleated giant cell, they are often the result of viral infections.(HIV can do this) can provide a network for viroid replication •tropism: cell, tissue, organ specificity. determined by the host cell receptor. complement, inner viron receptors, all examples. |
|
|
|
Viruses Can Evade Both Innate and Adaptive Immune Responses |
Innate –block or breakdown complement –block interferon production Adaptive –block antigen processing, MHC export –evade antibody -Antigenic Variation hemoglutanin eample) •amino acid changes in virion spikes (common in RNA viruses |
|
|
|
Mechanisms of Bacterial Pathogenicity |
Adherence: Invasion and spread: Colonization - establishing a site of microbial reproduction on or in host. Evading innate and adaptive immune responses |
|
|
|
Bacterial Adherence Factors |
-Capsid: Protein structure of a virus Capsule: shield barrier of pahthgen. adheres cells to a surface. (Streptococcus, Pneumoniae) |
|
|
|
Bacterial Adherence Factors continued... hnotes |
•Capsules •Examples of bacteria with capsules: vaccines for these are now available •Streptococcus pneumoniae •Haemophilus influenzae •Neisseria meningitidis • some strains of pseudomonas aeruginosa (opportunistic pathogen) |
Pyoverdin: siderophore (binds and grabs onto iron) can measure virulence |
|
|
Invasion and Spread |
•active penetration of host’s mucous membranes or epithelium •can be passive penetration –wounds, insect bites •once below mucous membrane, bacteria can spread to deeper tissues |
|
|
|
Table 35.4 is a list of microbial virulence factors involved in bacterial invasion and spread, I will expect you to know those on the list that we cover in lecture 7 total |
•Coagulase: produced by staphylococcus aureus (coagulates fibrinogen to clot. the clot protects pathogen from phagocytosis) •streptokinase: produced by streptococcus (binds to plasminogen, digests fibrin clots that allow pathogen to move freely) •Collagenase: produced by clostridium: (breaking down collagen that forms from framework of connective tissures) •hemolysins: in staph/streptococcui,ecoli. Lyse erythrocytes (make iron available for growth) •Immunoglobulin A protease: clostridium: cleaves immunoglobulin A into Fab and Fc frags. •leukocidins: staph,strept,pneumococci: pore forming exotoxins that kill leukocytes. decreases host resistance •deoxyribonuclease:clostridium perfringens: lowers viscosity, pathogen gains mobility. |
|
|
|
What is a NET? |
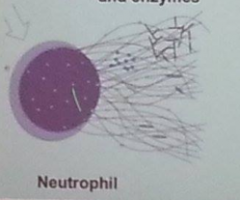
•Neutrophil Extracellular Trap: |
|
|
|
Colonization |
•Occurs when pathogen finds appropriate environment in host •Some bacteria invade specific cells • some bacteria found in the blood •Bacteremia: bacteria present in bloodstream
|
|
|
|
Bacterial Pathogens Can Evade Innate Immune Responses hnotes |
• Evading Complement: Capsules: inhibit opsonization by C3b or membrane attack complex formation. -Proteases to precent complement • lengthened O-side chains: one of the three components of LPS (gram negative) long O-chain can evade the innate immune response •evading cytokines: type III systems: they deliver proteins that block TLR signalling, and cytokine expression |
•Capsules •IgA proteases •antigenic Variation: change cell surface outer membrane or pili proteins |
|
|
Evading Phagocytosis |
•capsules •block phagocytic cells: streptococcus pyogenes M protein (blocks the process of phagocytosis) •Leukocidins: destroy phagocytes •proteases inactivate complement for opsonization |
|
|
|
Some Bacteria Survive In Phagocytic Cells |
•some microbes will prevent the lysosomes from digesting them. Some are resistant. like catalase. some microbes block the pathway in step 4. This is hard, ask a friend later Chris. |
|
|
|
Bacterial Intracellular Pathogens |
• Mycobacterium tuberculosis •legionella (legionnaires disease) •chlaymdia (obligate intracellular parasite) •listeria monocytogenes: causes listeriosis, is an intercellular pathogen. can infect canteloupe, gram+ foodborne pathogen, also psychrophile(grows in the cold) |
|
|
|
Bacteria can evade innate and adaptive immunity by forming biofilms |
Examples: •Pseudomonas in Cystic Fibrosis lung •Staphylococcus and Enterococcus on heart valves – endocarditis |
|
|
|
Bacteria can evade innate and adaptive immunity by forming biofilsm |
Cells + the matrix = biofilms -streptococcus pneumoniae (otitis Media) also known as inner ear infection. biofilms are formed within the ear -Pseudomonas in Cystic Fibrosis in the lung, also can form biofilms. colonies embeded in the matrix -Staphylococcus and enterococcus have made biofilms on heart valves (endocarditis) |
|
|
|
Toxins |
•Substances that damage host –Exotoxins (secreted from cells) –Endotoxins (poisons within the cell itself 4 types of Exotoxins: slides shown later -membrane disrupting -super antigens -AB exotoxins (2 portions) -Specific Host site |
|
|
|
Membrane-Disrupting Exotoxins |
-Pore-forming exotoxins (leukocidins, Hemolysins)
|
|
|
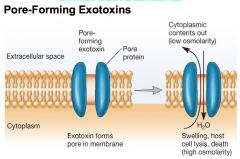
|
hemolysins are also pore forming bacteria that cause complete hemolysis. |
|
|
|
SUPERANTIGENS: binds to the Tcell receptor and the antigen MHC II, locking them together. Helper T-cell becomes super activated. Cause Tcells to overexpress (over 30%) Leads to the failure of multiple host organs causes Toxic Shock syndrome, caused by staphylococcus aureus superantigen |
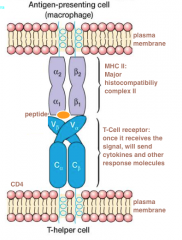
|
|
|
|
AB Exotoxins |
Two subunits (A and B) A- has a toxic effect B- binds to a target cell receptor |
|
|
|
Diphtheria Toxin Corynebacterium Diphtheriae hpic |
Enters by receptor-mediated endocytosis. Binds to the epidermal growth factor receptor. -lowering of the pH dissociates the A subunit from the B subunit (separates them) A-subunit functions as an ADP ribosal transferase. Attaches ADP ribose from NAD onto EF-2 (elongation factor, helps with protein synthesis during translation) |
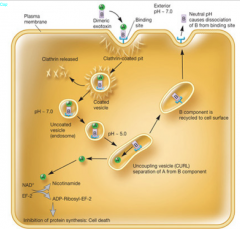
|
|
|
Specific Host Site Exotoxins 2 examples hnotes |
Haiti Cholera: 500,000 infected. -vibrio cholerae: produces enterotoxin(intestine related) also an ADP-ribosyltransferase, modifies host G-protein(Gprotein controls adenylate cyclase, that increases cAMP "cyclic AMP") this alters Na and Cl transport, leads to H20 loss Diphtheria and Cholera toxin genes are encoded by lysogenic prophage |
-Clostridium Botulinum: gram (+) spore forming bacterium. (causes flaccid paralysis) Neurotoxin- blocks acetylcholine release at neuromuscular junctions (stops them from contracting, forever staying flaccid/limp) |
|
|
Exotoxins are proteins that are often antigenic hpic |
-Antibody (antitoxin) can neutralize toxicity -exotoxins are generally unstable, can lose toxicity but remain antigenic toxoid: inactivated toxin that can still elicit an immune response. (part of DTaP vaccine, often used for vaccines) "Diptheria tetanus acelluar P-something" |
|
|
|
Endotoxin hpic |
-Lipid A of LPS (lipopolysaccharide) Effects are often indirect b/c of TLR4: stimulates endogenous pyrogen from macrophages. Also stimulates lots of macrophages, makes lots of cytokines, activates TNF (tumor necrosis factor) to release. Becomes uncontrolled in amounts leads to capillary leak and hypotension (low blood pressure) finally gram (-) shock, organ failure |
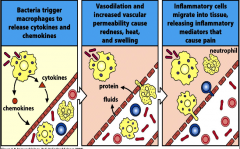
|
|
|
Type III Secretion Systems: Hand to Hand Combat |
Def: when there is direct injection of toxins -So far only done by Gram (-) pathogens for human and plants -inject Effector proteins: invasion, control host immune response and intracellular survival (parasitic) Injectisome: the process of effector proteins being shot through the needle |
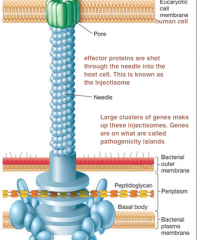
|
|
|
Targets for Type III Effectors |
1)Host cell cytoskeletons: actin (enteropathogenic Escherichia coli induces pedestal formation on host cells.) 2)Host cell signalling pathways: (NFkB) is targeted and blocked. Now the cell cant send out cytokines and other response proteins |
|
|
|
Enteropathogenic E. coli (EPEC) Type III Secretion System |
•EPEC - major cause of infantile diarrhea, Binds host cells using bundle forming pili. they delivers effector proteins, including Tir (translocated intimin Receptor) which ends up on the surface of the human cell, and binds to bacterial intimin. |
|
|
|
Microbial Control - Terms |
•CIDAL – kills •STATIC – inhibits growth •STERILIZATION – completely remove or kill all microbes •DISINFECTION – reduction of microbial population, destruction of pathogens •SANITIZATION – reduction of microbial contamination to levels safe by public health standards •ANTISEPTIC – chemical agent applied to tissue to prevent infection by inhibition or killing |
|
|
|
Microbial Control Methods |
•Mechanical Removal – filtration •Chemical agents – gases •Physical agents – radiation, heat |
|
|
|
Antimicrobial Agents
|
•used to treat disease
•destroy pathogenic microbes or inhibit growth •most are antibiotics–microbial products that kill or inhibit |
|
|
|
Microbial Sources of Some Antibiotics |
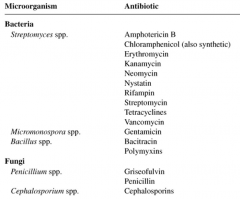
|
|
|
|
Development of Antimicrobial Therapy |
•Paul Ehrlich (1904) - selective toxicity •Alexander Fleming (1928) - accidently rediscovered penicillin •Florey, Chain, and Heatley (1940) –purified penicillin, when injected into mice infected with Staphylococcus, mice survived •Selman Waksman (1944) –cultured over 10,000 strains of soil bacteria and identified streptomycin |
|
|
|
List of antibacterial drugs and properties – many we will cover in lecture |
broad-spectrum: attack many different pathogens
narrow-spectrum: attack only a few different pathogen |
|
|
|
Considerations For Developing a Clinically-Useful Antibiotic |
"fell asleep" |
|
|
|
Mechanism of Action of Antimicrobial Agents |
•Targets: –Cell wall –Plasma membrane –Nucleic acid synthesis –Protein synthesis –Metabolic enzymes |
|
|
|
Bacterial Cell Wall Synthesis Is a Major Target for Antibiotics |
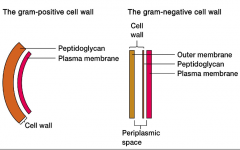
|
|
|
|
Cell Wall Inhibitors |
"still asleep" |
|
|
|
Penicillins inhibit bacterial Penicillin Binding Proteins (PBPs) used for transpeptidation (hnotes |
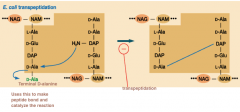
|
|
|
|
Other Cell Wall Inhibitors |
Penicillins and Cephalosporins - Beta lactam structure Vancomycin: antibiotic resistnant microbes have changes the D-alanine to a different acid Bacitracin: blocks that carrier that moves subunits across the plasma membrane |
|
|
|
Plasma Membrane Inhibitors |
•Polymyxins - disrupt lipid bilayer (cidal, narrow spectrum, effective against gram negatives) ex: Colistin (comes from bacilus, gram (+)) •Antifungal agents often target plasma membrane. (Miconazole) blocks sterol synthesis. "Candida" yeast and infections and athletes foot (caused by Tinea pedis) •Nystatin: binds sterols (like Candida yeast infections) •Some target the cell wall itself (Nikkomycin) blocks the synthesis of Chitin. |
|
|
|
Nucleic Acid Inhibitors |
•Quinolones: (Nalidixic Acid, Ciprofloxacin) - Cidal, broad spectrum - Synthetic - Bind DNA gyrase (DNA replication) thats in charge of underwinding ahead of the DNA replicator. •Rifampin -Cidal, broad spectrum -binds bacterial RNA polymerase |
|
|
|
Protein Synthesis Inhibitors hnotes |
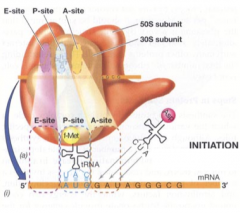
•take advantage of differences between eukaryotic and prokaryotic ribosomes and protein synthesis machinery |
|
|
|
Protein Synthesis Inhibitors
|
•aminoglycosides (cidal): bind the 30s, cause mRNA misleading. examples: streptomycin, and kanamycin.
•Tetracyclines (static): bind the 30s, distorts the A-site, inhibits aminoacyl tRNA binding •Macrolides (static): bind the 23S rRNA of the 50s, blocks peptide elongation. examples are erythomycin, and azithromycin. this disrupts protein synthesis in bacteria like chlamydia •Chloramphenicol: same MOA as Macrolides. Toxic, non-life threatening |
|
|
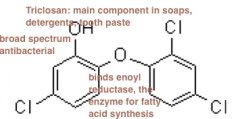
Metabolic Enzyme Inhibitors hnotes |
•Sulfonamides and Trimethoprim: (synthetic, static) are examples of Antimetabolites: disrupt metabolic pathways of the bacterial pathogen. Similar to metabolic intermediates (AKA analogs) if you have a metabolic pathway, and enzymes that are part of that substrate, the enzyme will instead bind to the drug rather than the substrate |
•Also binds enzymes for folic acid synthesis, folic acid in turn blocks bacterial purine, pyrimidine synthesis. •by messing with the enzymes involved with folic acid, stops aminoacid synthesis. (as humans we get our folic acid from our food rather than self made) |
|
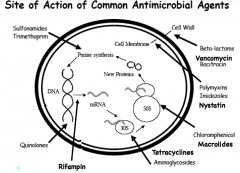
|
Beta-lactams: all part of the synthesis of peptidoglycan. Vancomycin stops trans-peptidation, stopping peptidoglycan synthesis. also , bacitracin blocks the carrier so that they cant build in the cytoplasm Imidazoles and Nystatin: anti fungal enzymes Chloramphenicol and macrolides: disrupt the 23S from connecting Tetracyclines and aminoglycosides: disrupts and distarts 30S from working. Rifampin: stops transcription, blocks DNA from making mRNA Quinolones: blocks DNA gyrase Metabolic enzyme inhibitors, like sulfonamides and trimethopim inhibit metabolic stuff. |
|
|
|
Antibiotic resistance is prevalent in an isolated cave microbiome |
In new mexico, in lechuguilla cave, isollated for 4 million years, they found microbes that were highly resisntant to 14 different normally used antibiotics. This is proof that antibiotic resistance is a long part of microbe history and genetics. |
|
|
|
Antiviral Drugs
|
There are few Antiviral drugs, why? because viruses normally use the host DNA, so we'd die.
Examples of them: Acyclovir: inhibits DNA polymerase of herpes viruses (guanine analog) Tamiflu: blocks neuraminidase (ex: influenza) |
|
|
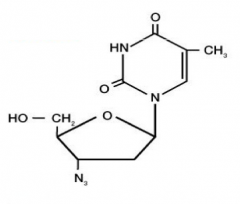
Anti-HIV Drugs |
-HAART (Highly active Anti retroviral therapy) -Azidothymidine (AZT): targets reverse transcriptase, also called nucleoside analog, causing DNA chain termination -the N3 group at the bottom blocks reverse transcriptase of viruses -Ritonavir (protease inhibitor): this inhibits HIV protease that processes viral proteins for virion assembly |
|
|
|
Antiprotozoan Drugs |
-Malaria: Chloroquine, Malorone are drugs that stop malaria -Metronidazole: treats Giardia and Trichomonas protozoan parasites, also treats infections of Clostridium and Helicobacter (both anaerobic and microaerophillic bacteria) Metronidazole enters the parasite, activated by reduction, and interacts with DNA by "nicking" cutting it up. |
|
|
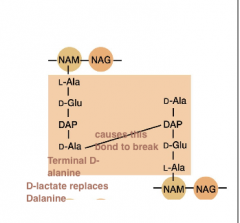
Superbugs |
-Vancomycin resistant Enterococcus (VRE): has a vanA gene that encodes enzyme to replace D-alanine with D-lactate -Methicillin resistant Staphylococcus aureus (MRSA): has the mecA gene, encodes PBP that is resistant to penicillin |
|
|
|
Mechanisms of Drug Resistance |
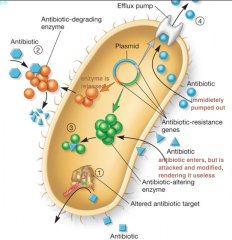
|
|
|
|
Overcoming Drug Resistance |
•Antibiotics don’t mutate microbes, but rather create environments that select for antibiotic-resistant mutants. •use drugs only when necessary, take prescribed course •don’t treat viral infections with antibacterials •give drug in high concentrations •give two or more drugs at same time: must give penicillin and clavulanic Acid at same time, the acid blocks the anti-penicillin enzymes in bacteria |
|
|

Identification and Development of New Antimicrobials hnotes |
Listex: is a culture of safe micro-organisms (bacteriophage preparation) in use as a processing-aid characterized by its broad spectrum to treat Listeria monocytogenes |
|
|
|
New leads? (ON THE TEST) |
-Red Alga: Genus: Delisea prefers that microbes to not make biofilms on it. Makes furanones -Furanones: blocks bacterial biofilm formation "inhibits AHL mediated QS" Acyl-homoserine Lactone mediated Quarum sensing system AHL is an autoinducer. |
|
|
|
Viral Diseases |

|
|
|
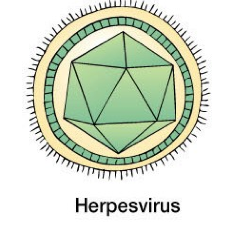
Chickenpox |
-Caricella-zoster virus: causative agent of Chickenpox. Family: Herpesviridae They are envelope viruses. Often are icosahedral morphology. their genomic material is DNA Often transferred via Inhalation or conjunctiva of the eye(rubbing the eye) virus spreads via blood, infects lymphatic and neuronal system -after 10 days infection of skin leads to vesicular rash Treatment: drug? attenuated (live)vaccine |
|
|
|
Vaccines -Inactivated(killed) vaccines: use chemicals or heat used to kill virus. Can induce humoral immune response (b-cells) DRAWBACK: requires booster (not long lasting) ex) rabies and flu shot -Attenuated (live but avirulent): inactvate specific genes in virus. can reproduce in your cells, but weakened, and by doing so helps create humoral response and cell-mediated immune response. Drawback: may revert to virulent chickenpox, MMR(measles, mumps, rubella), Flu |
Herd Immunity: •Protection of unvaccinated people in a population where most people are vaccinated due to lessened risk of disease transmission |
|
|
|
Shingles (Zoster) hnotes |
•individuals who recover from chicken pox are often resistant to disease •however, viral DNA can reside in dormant state in nuclei of nerves, sensory neurons –Latency •immunocompromised state (age, organ transplant, AIDS, stress) can reactivate virus - leading to Shingles |
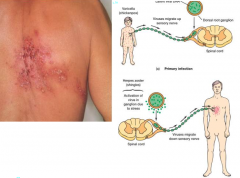
Shingles: when chickenpox(or other Caricella-zoster viruses) is reactivated in a grownup. |
|
Influenza (Flu) |
-negative ssRNA virus: made of segmented genome. -must bring its own RNA replicase to convert its negative ssRNA into positve ssRNA. does this so it can utilize host ribosomes. -Enters cells via endocytosis (within an organelle) and exits(releases) from cells via budding
|
|
|
|
Changes in Antigenicity of Influenza Viruses hnotes |
Antigenic drift: refers to minor changes in the antigenicity of a virus. mutations in a viral gene in a single strain. -largely driven by the rival RNA replicase, which are error-prone. some of these errors weaken the virus, others strengthen them by luck |
Antigenic Shift: refers to major changes in antigencity of virus. -Different strains (animal or human) can infect cell, and cause genomes to reassort. basically takes viral genome from multiple animal sources, causes recombination, creates more virulent strain. animal influenza: zoonotic Epidemic: sudden increase in disease Pandemic: increase in large geographically widespread pop. 1918 spanish flu pandemic (duck, pig, human) |
|
|
Measles, Mumps, Rubella |
-RNA viruses -MMR attenuated (live) vaccines are needed. |
|
|
|
Arthropod-Borne Diseases (often mosquito transmitted) |
-yellow fever -west nile fever -Dengue fever- 400 million infected yearly in the tropics (peurto rico), are subtropic all caused by Flaviviruses: are positive ssRNA, enveloped and have icosahedral morphologies. |
|
|
|
Direct Contact Diseases: Common Cold, Mononucleosis, AIDS and Ebola |
Common cold: major cause- Rhinovirus (rhino = nose) -ssRNA, naked (no envelope), icosahedral shape -over 100 different serotypes (reason why we dont have a vaccine for it, too many types) |
|
|
|
Mononucleosis (Mono) |
-caused by Epstein-Barr virus (family herpesviridae) -These are DNA viruses -icosahedral capsule morphology (has envelope) -enters and replicates in the epithelial cells of the throat (kissing disease) -infects B cells (can enter a latent stage, enters genome, replicate and mess with you later) -uses MHC II cells as a receptor for the molecule -Causes Cancer (Burkitt's lymphoma) - primarily in Africa, in children with malaria |
|
|
|
Warts |
•Human Papillomaviruses (HPV) –naked, icosahedral, DNA –>100 different strains –Genital HPV - most common sexually transmitted infection –some are oncogenic - cervical cancer •How do viruses cause cancer? (Textpgs125-6) Viral protein E6 targets destruction of host "p53" "E" stands for early protein. |
|
|
|
Acquired Immune Deficiency Syndrome (AIDS) |
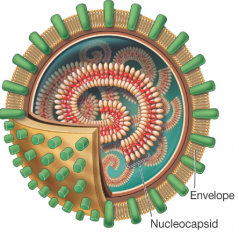
•Human immunodeficiency virus (HIV) –Retrovirus –Enveloped, RNA genome –Partly icosahedral, cone-shaped core –Originated in Africa from non-human primates (HIV-1 - chimpanzees) |
|
|
|
HIV hpic |
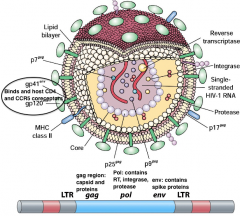
|
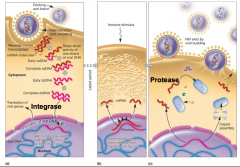
|
|
|
HIV Transmission and Pathogenesis |
•When infected blood, semen, or vaginal secretions come in contact with uninfected person’s broken skin or mucous membranes (sexual contact, transfusion, needle sharing) •Pathogenesis –T cell depletion,also macrophages, dendritic cells –virus mutates rapidly, evades immune system (due to error prone reverse transcriptase) |
|
|
|
Acquired Immune Deficiency Syndrome (AIDS) |
CDC definition: HIV infected individual with <200 CD4+ Tcells/microliter of blood T Cell destruction -> immune system collapse -> opportunistic infections •presently,no cure for AIDS, treatment directed at: –Reducing viral load (HAART) –Treating opportunistic infections and malignancies (Kaposi’s sarcoma) -- latency is a challenge, can enter latent stage in helper T cells, meaning it will always integrate –Education key to prevention and control |
|
|
|
Ebola Hemorrhagic Fever |
Ebola virus: -ssRNA, filamentous, enveloped (RNA replicase makes the ssRNA abundant) family: filoviridae viral proteins block interferon, also causes the clotting of blood, causing hemoraging Transmission: direct contact with blood or body fluids of infected symptomatic person. Airborne transmission hypothesized, but not demonstrated at this time in humans evidence zoonotic- fruit bats, primates treatment: mostly supportive, just suffer and live |
outbreak in 2014 in Africa, currently 15,351 cases, and 5,459 deaths. |
|
|
Food and Water-Borne Viral Disease - Gastroenteritis |
-Rotavirus -Noro (norwalk-like) virus (hit a couple hundred people at a post-oscar party in the seafood) -both Rota and Noro are naked RNA viruses. -Fecal-oral and also person-to-person (projectile vomit) |
|
|
|
Polio (infantile paralysis) |
•Poliovirus (enterovirus, +ssRNA) –stable food, water (ingestion) –multiplies throat, intestinal cells –targets motornerve cells in spinal cord - paralysis •Vaccines: Salk (killed) and Sabin (live, oral) |
|
|
|
Viral Zoonotic Diseases |
-spread between animals and humans -rodents, amphibians, reptiles, insects, domestic and wild animals causes: viruses, bacteria, fungi, protozoa -75% of recently emerging infections diseases affecting humans are diseases of animal origin |
|
|
|
Rabies |
Rabies virus: bullet shaped, enveloped virion RNA) -multiplies in animal salivary glands targets the muscle cells (tropism for muscle cells) spreads via CNA (central nervous system) to brain (negri bodies) - paralysis |
|
|
|
Bacterial diseases! |

|
|
|
|
Tuberculosis (TB) hnotes |
-Mycobacterium tuberculosis acquired- inhalation. lung phagocytosed, survive intracellularly mycolic acids in cell wall- for protection host responds with tubercles -latency in response to signals, tubercles can liquify, bacteria spread to blood, now it can spread to blood, goes to organs, causing death |
TB Skin Test •The Mantoux Tuberculin Skin Test is standard method of determining whether a person has had TB or been exposed to M. tuberculosis -performed by injecting purified protein into forearm. reaction (induration) measured in mm (48-72 hours) -reaction: delayed hypersensitivity - memory T cells -tubercles and granulosa are basically the same thing, a small mass of macrophages |
|
|
TB Skin Test |
•Care must be taken in interpreting •False Positives –Infection with nontuberculosis Mycobacterium –Previous BCG vaccination –Incorrect administration or interpretation •Follow up: Chest X-ray, bacterial culture,microscopy with acid-fast staining |
|
|
|
TB – Vaccine, Diagnosis and Therapy |
•BCG Vaccine -BacilleCalmette-Guerin –live avirulent M. bovis •Diagnosis –bloody sputum, chest x-ray, acid-fast staining, culture -antimicrobial therapy: Rifampin + Isoniazid Isoniazid targets mycolic acid synthesis drawback, these therapys are required daily for 6-9 months. Rifampin targets RNA polymerase, transcription -MDR and XDR and strains emerging (multi drug and x-treme drug resistant) |
|
|
|
Streptococcal Diseases |
•Streptococcus pyogenes (group A β hemolytic) or S.pneumoniae •Infections - impetigo (skin), throat and lung infections, pneumonia otitis media( inner ear infection) •Diagnosis - strep test, culture •Treatment - penicillins, erythromycin |
|
|
|
Streptococcal Virulence Determinants |
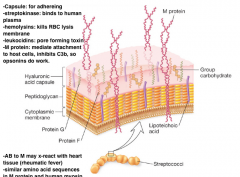
|
|
|
|
Group A streptococci can also cause invasive infections |
-Some strains make tissue destroying protease -Necrotizing fascititus: makes flesh eating bacteria
|
|
|
|
Streptococcus is also associated with tooth decay |
-"Streptococcus mutans" -part of the dental plaque (biofilm) -Fermentation occurs-> utilizes sugars to make acid --> causes enamel decay |
|
|
Whooping cough (pertussis) |
-Bordetella pertussis (gram -) -Colonizes ciliated cells of respiratory tract -stage 1: cold-like symptoms -stage 2: prolonged paroxysmal cough (spasm) -virulence factors: type III secretion, uses pilli to attach, siderophores(use of microbes to obtain iron) -PTx: AB exotosin, same MOA(mode of action) as cholera Toxin. ADP ribosal toxinase |
|
|
|
Pertussis Vaccination and Treatment |
•Subunit Vaccine – DTP 1940’s (Diptheria, Tetanus, pertussis) •DTaP (currently): Toxoids, the "a" stands for acellular. •Tdap booster •Antibiotics -tetracycline, erythromycin (protein synthesis inhibitors) |
|
|
|
Meningitis Outbreaks in Princeton, UC Santa Barbara prompt concern. vaccine efforts |
State law requires all princeton students to receive a meningitis vaccine, but it doesnt protect against the type B strain, Federal officials have taken the rare step of allowing the university to vaccinate thousands of students using a vaccine not yet approved in the US. |
|
|
|
Meningitis |
•Inflammation brain, spinal cord meninges (membranes) •bacteria, viruses, fungi (comes in all categories) •bacteria –Haemophilus influenzae –Streptococcus pneumoniae –Neisseria meningitidis |
|
|
|
Neisseria meningitidis |
-leading cause meningococcal disease in children and young adults -person to person, respiratory or throat secretions -can cross mucosal barrier into blood -Serogroups: groups of strains with common surface antigens (A, B, C, Y, W) -virulence factors: Pilli, capsules, endotoxin (gram -) |
|
|
|
Neisseria meningitidis Meningitis |
•Clinical - initial sore throat, vomiting, confusion, stiffness in neck, rash •Prevention (Vaccines): MCV4, capsular polysaccharide. protects against A, C, Y, W, but not B. MenB: group B outer membrane proteins •Diagnosis, Treatment –gram stain spinal fluid, culture –antibiotics (cell wall, protein synthesis) |
|
|
|
Arthropod-Borne Diseases |
–Plague –Lyme Disease |
|
|
|
The Black Death or Plague spread throughout Europe during the Middle Ages, claiming over 30 million victims |
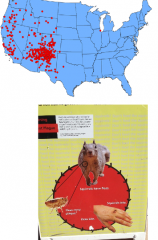
|
|
|
|
The Plague (black death) |
- Yersinia pestis (Gram - ) -2 forms: bubonic, spread by fleas. pneumonic (person to person): flu-like symptoms, nearly 100% fatal -Category A Bioweapons Agent |
|
|
|
TYPE III secretions |
Type III Secretion is a major virulence factor of Yersinia. Type III injectisome delivers effector proteins called YOPs (yersinia outer proteins) into host cells including macrophages. needle like structure, inserts proteins. |
|
|
|
Lyme Disease |
•Most commonly reported tick-borne disease in US -Caused by spirochete Borrelia Burgdorferi -vector = blacklegged (deer) tick - Ixodes |
|
|
|
Stages of Lyme Disease |
-Localized (7-10 days): bulls-eye rash develops (erythema migrans) flu like symptoms. most treatable with doxycycline (a tetracycline) and penicillins •disseminated (weeks or months): causes muscle pain, and arthritic symptoms (autoimmune response) –muscle pain, arthritis (autoimmunity?) •late (years): nervous system: |
|
|
|
Direct Contact Diseases |
–Anthrax (zoonotic) –Staphylococcal Diseases |
|
|
Anthrax |
-Caused by Bacillus anthracis (spore forming) -virulence factors(capsules, exotoxin -AB but 3 parts. The toxin and capsule encoded on separate plasmids) both are required to become afflicted. |
|
|
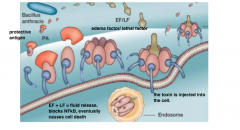
Anthrax Toxin |
Forms of Anthrax -Cutaneous: cuts or abrasion -pulmonary anthrax: inhaled by spores: fatal if bacteria reach the bloodstream -bioterrorism (2001) sent by envelope to president. bioattack -treatment: ciprofloxacin- blocks the anthrax's ability to replicate. DNA gyrase inhibitor |
|
|
|
Staphylococcal Diseases |
•Members of the gram-positive genus Staphylococcus are among the most important bacteria that cause disease in humans •Two major species –S. aureus -invasive, virulent (coagulase +) causing blood to coagulate at site of infection –S.epidermidis: less invasive, less virulent (coagulase -) Diseases include: boils, carbuncles, toxic shock syndrome, food poisoning |
|
|
|
Staphylococcus aureus Has An Arsenal of Virulence Factors |
he didnt say anything really... |
|
|
|
Food and Water-Borne Bacterial Diseases |
•Cholera •Listeriosis •Botulism •Escherichia coli and Salmonella typhimurium infections •generally prevented and controlled by sanitation measures, antitoxins, and antibiotic therapy |
|
|
|
E. coli 0157:H7 |
-Enterohemorrhagic E. coli (EHEC) -the infectious dose is <100 cells (very potent) -carriers- cattle, swine -shiga-like toxin- its an AB toxin: binds to glycolipid receptors on kidney and intestinal cells and enters -this toxin cleaves rRNA -hemolytic uremic syndrome, toxin genes on the prophage |
|
|
|
Fermented: Foods not only serve as important vehicles for disease transmission, but can be transformed by microbes into gastronomic delights |
Microbiology of Fermented Foods –microbial fermentations play an important role in the food industry including the production of yogurt, cheese, chocolate and alcoholic beverages - chocolate = numerous microbial fermentations >300 compounds –the major fermentations used are the lactic, propionic and ethanolic fermentations |
|
|
|
Fermentation: Consequence of life without air |
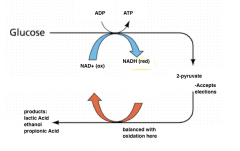
|
|
|
|
Yogurt Production - Lactic Acid Bacteria |
•Lactobacillus + Streptococcus -starter culture (form a symbiosis in yogurt between the lactobacillus and streptococcus) •Lactose (in milk) hydrolyzed to glucose, which is then fermented to lactic acid |
|
|
|
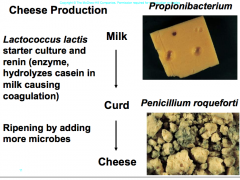
Cheese Production |
-Lactococcus lactis starter culture and renin (enzyme, hydrolyzes casein, coagulation) this forms curd, then by ripening (adding more microbes) makes curd into cheese. propionibacterium: makes cheese (swiss cheese, makes the gas that makes the holes) Penicillium roqueforti: blue cheese from a fungus
|
|
|
|
Plastic and Lactic Acid Fermentation |
-polylactide (natureworks) bio-based plastic -uses dextrose from corn fermented to lactice acid, purified, then polymerized. -made to make cups - bioengineered E. coli, lactobacillus does this fermentation |
|
|
Extreme Probiotics (TEST) |
Bacterial Transplant Succeeds U of M Physician Dr. Alexander Khoruts and his team have begun transplanting bacteria from a donor's healthy colon to combat raging Clostridium difficile infections. His team has performed 16 successful colonic bacterial transplants to combat refractory Clostridium difficile, a crippling infection responsible for at least 5,000 deaths/year. Procedure involves injecting a donor’s fecal material into the colon of the infected patient. Symptoms associated with the infection are relieved and infection eliminated. The researchers showed that donor bacteria can become dominant resident flora in a new adult host |
|
|
|
RESEARCH |
Researchers are studying how to improve diabetes patients’ insulin sensitivity by transplanting trillions of beneficial bacteria into their intestines to improve how the body regulates blood sugar, the central problem in diabetics. To better understand how microbial balance affects communication between the brain and gut, before and after transplant researchers will scan patients’ brains while showing them pictures of food, recording the brain response triggered by microbes in the gut |
What does microbiology have to do with beer? •“In a word – yeast. Without yeast, there would be no beer. It’s that simple. And yeast is a microbe, that is, a living organism that is too small to see with the human eye. Every time someone brews a batch of beer, in a very real sense he or she is doing a microbiology experiment. If you brew beer at home you’re a microbiologist! (In fact, you might even want to join the American Society for Microbiology!). So let’s talk about yeast” |
|
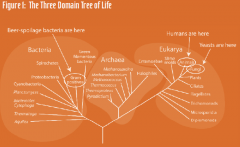
|
HOW IS BEER MADE? -Malting and mashing the grains, plant enzymes breakdown complex starches, proteins. add Hops, and Saccharomyces are added |

|
|
|
Biofeuls: fuels from bio-material |
|
|
|
|
Industrial Microbiology |
Microbial growth and product production occurs in bioreactors that can hold over 100,000 liters |
|
|
|
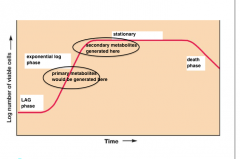
Metabolic products made by microbes fall into two general categories |
Primary metabolites Required for growth Made during active growth Example: amino acids Secondary metabolites Not required for growth. Made under nutrient limiting conditions following active growth Example: antibiotics |
|
|
|
empty slide |
|

